Study on the role of CaF2 in CaO-Al2O3-based mold fluxes with different acid-base property
-
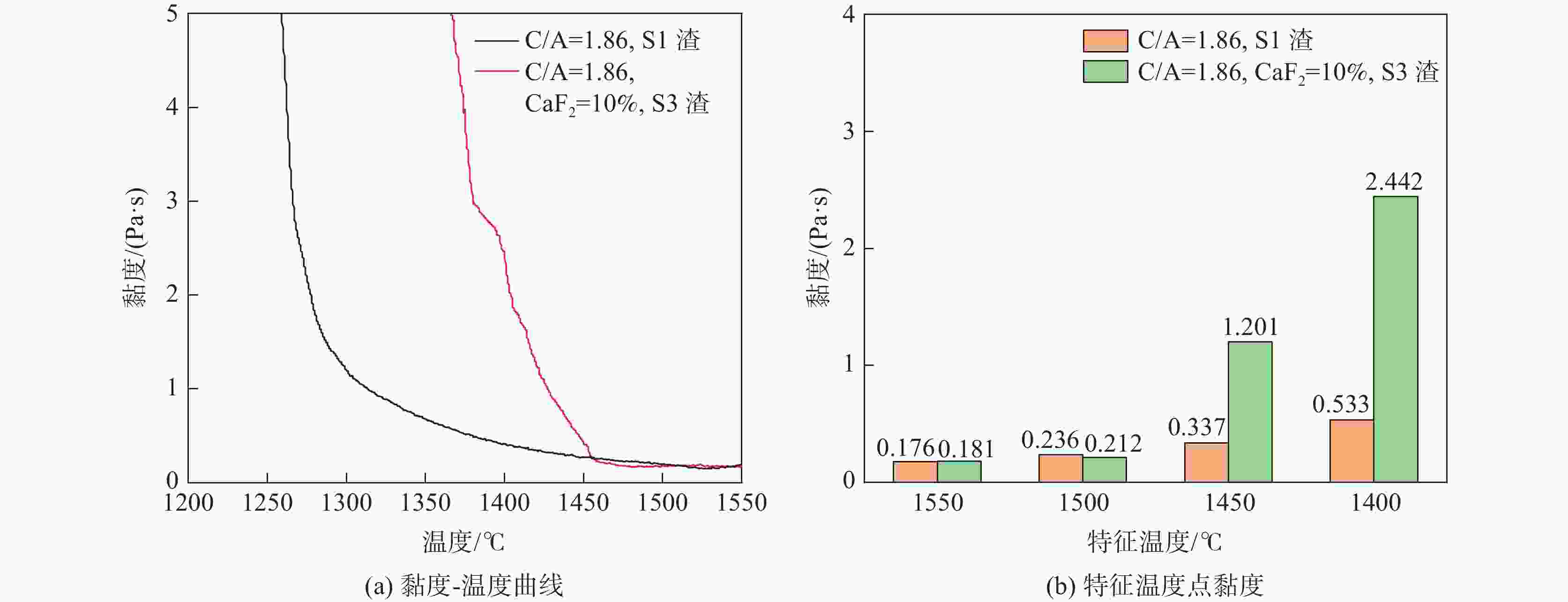
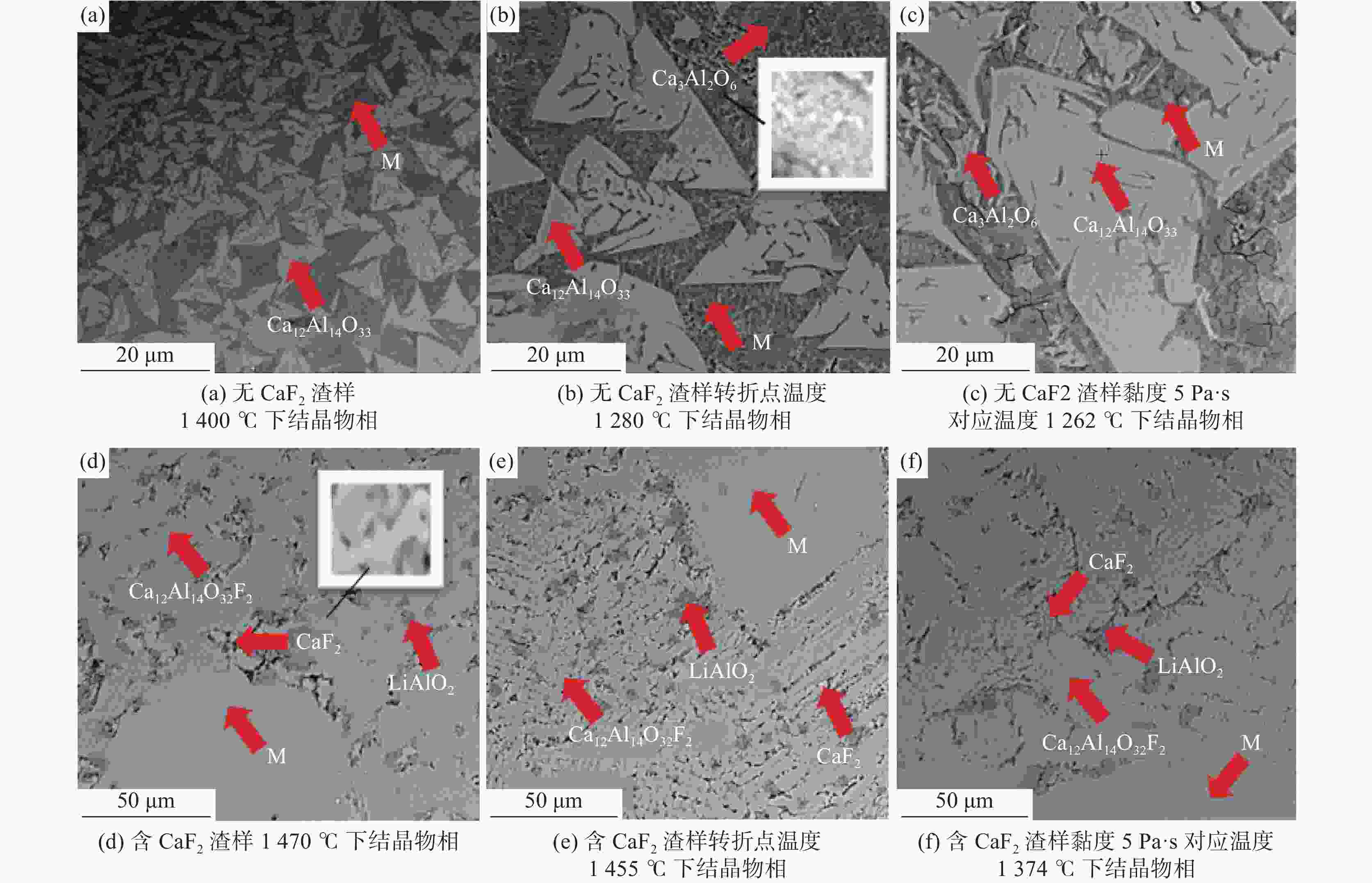
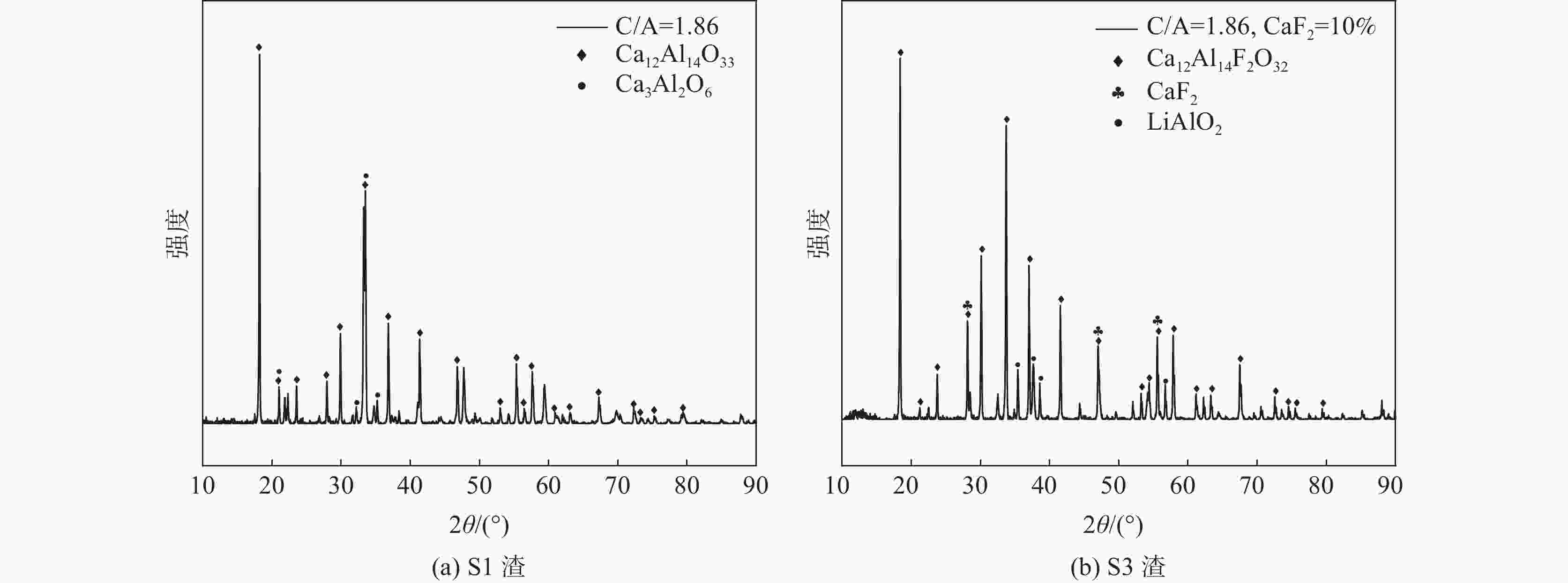
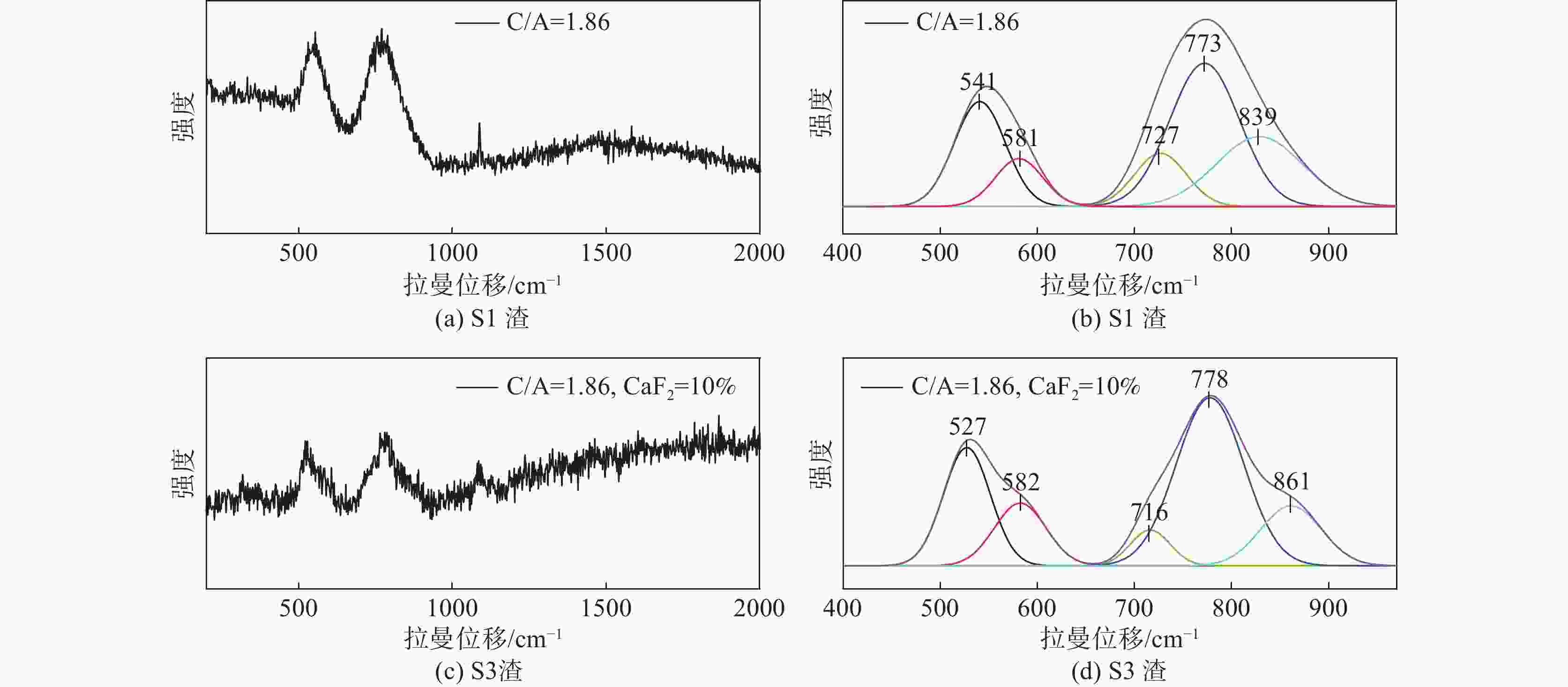
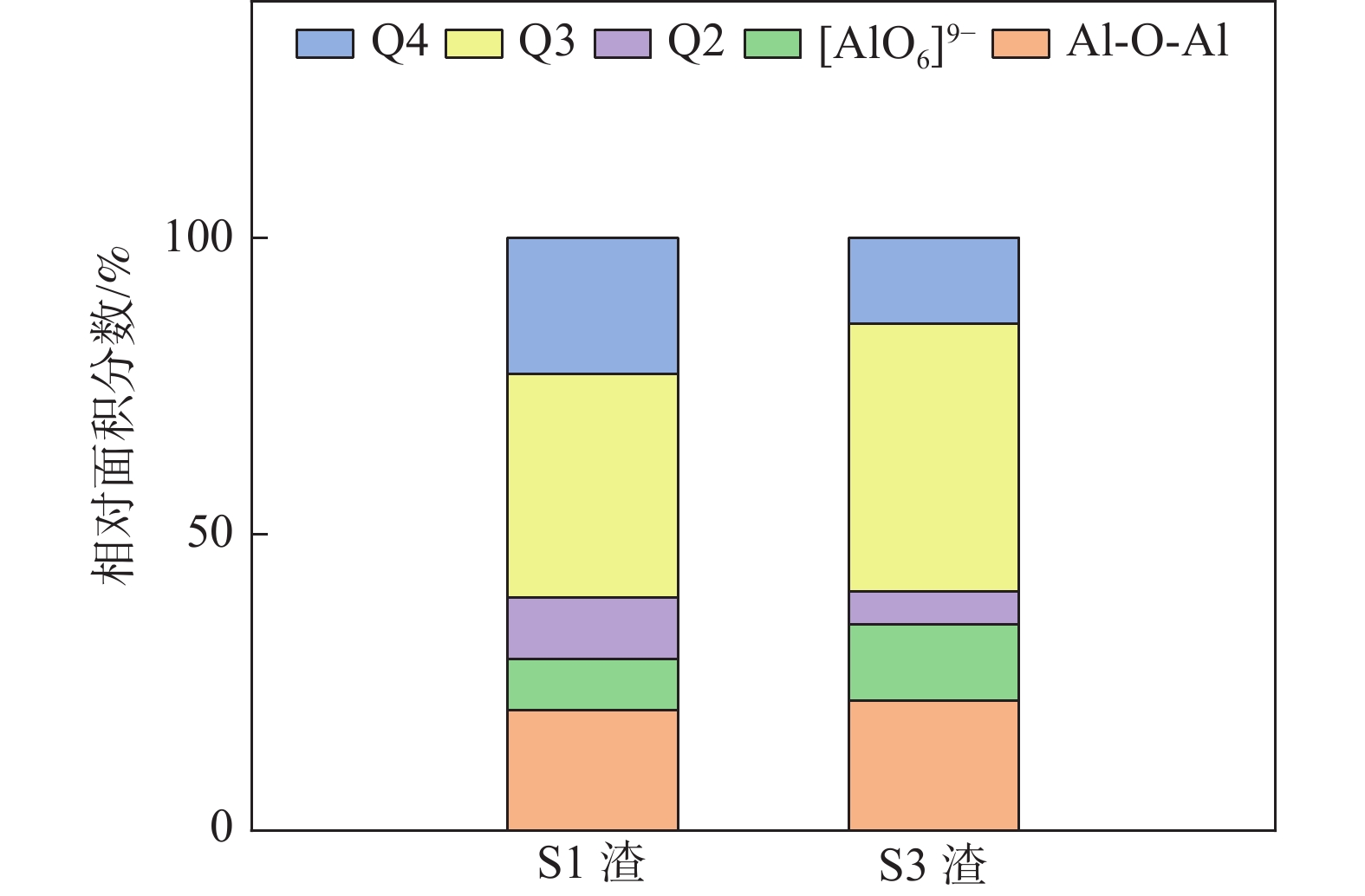
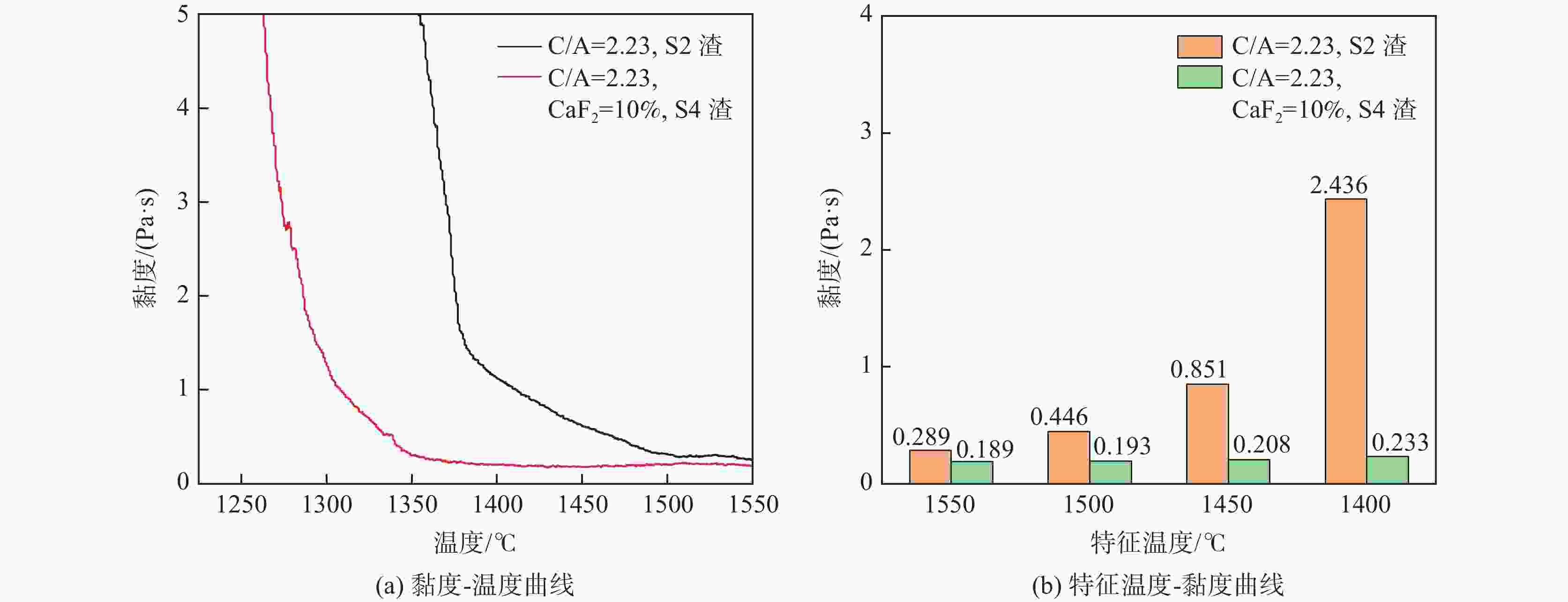
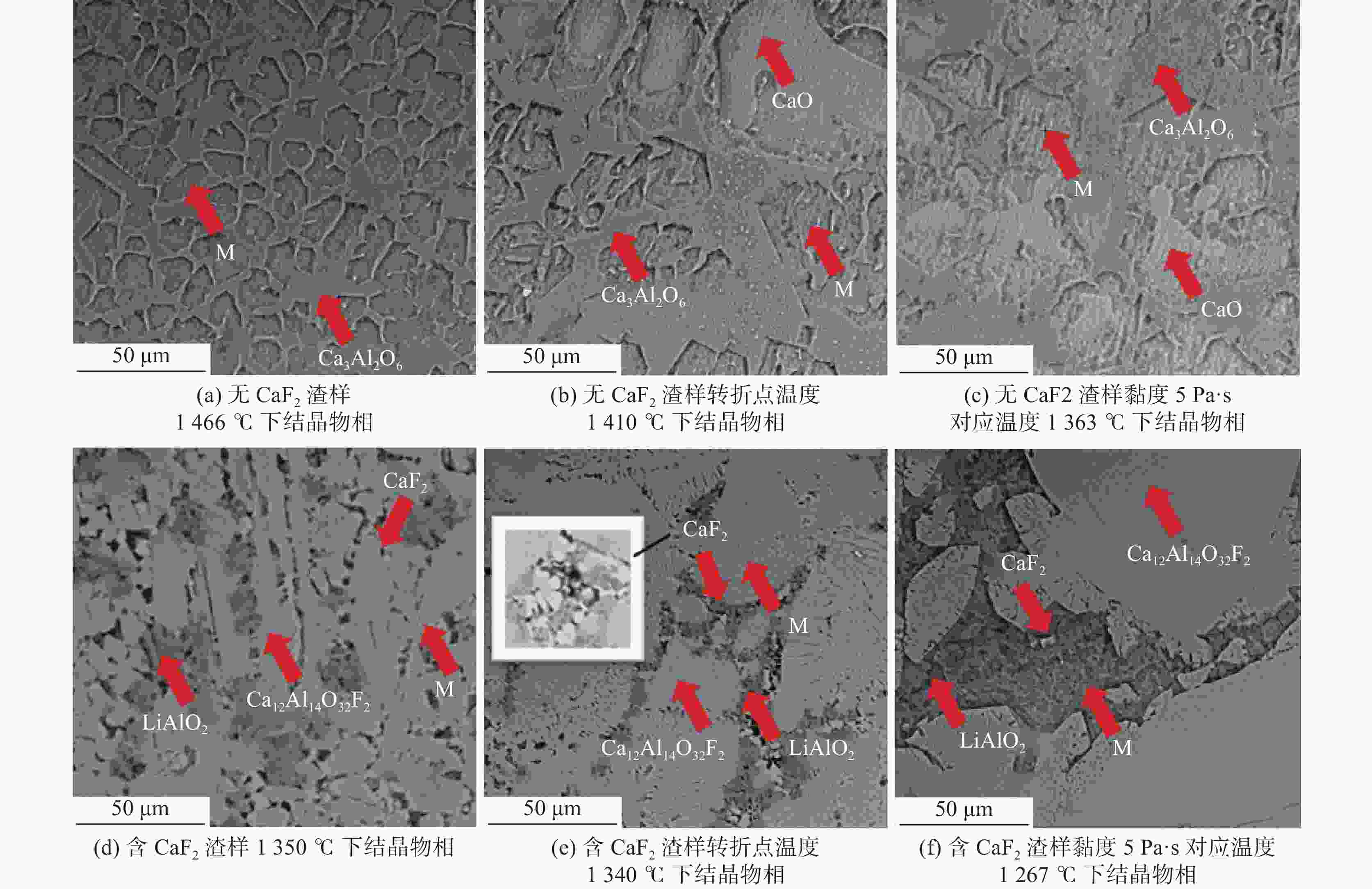
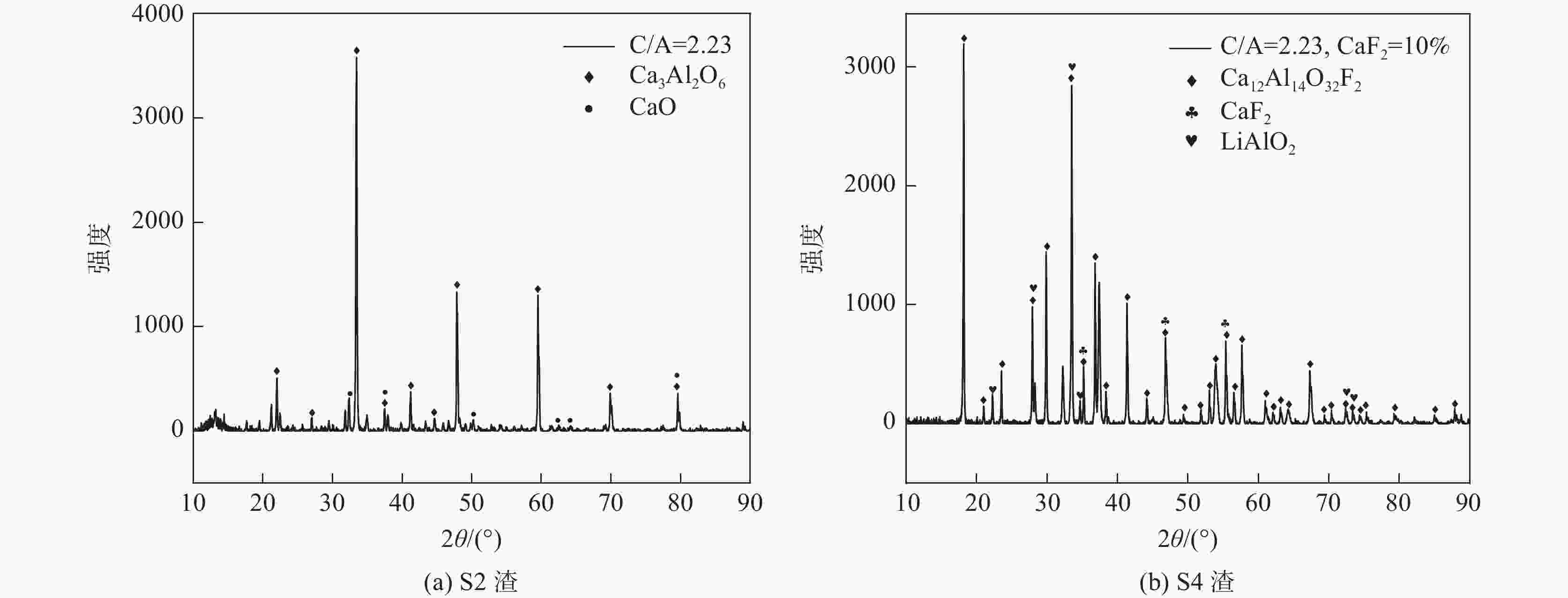
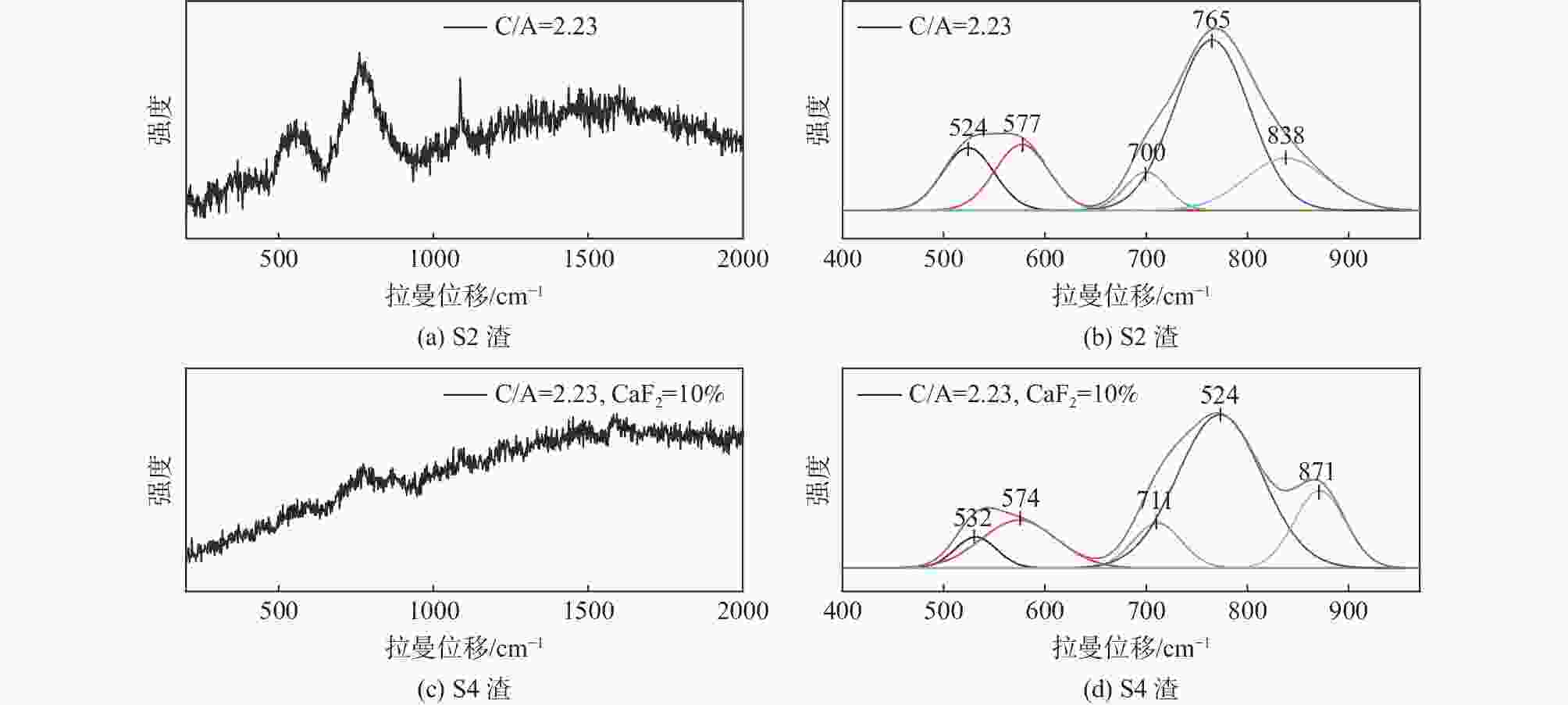
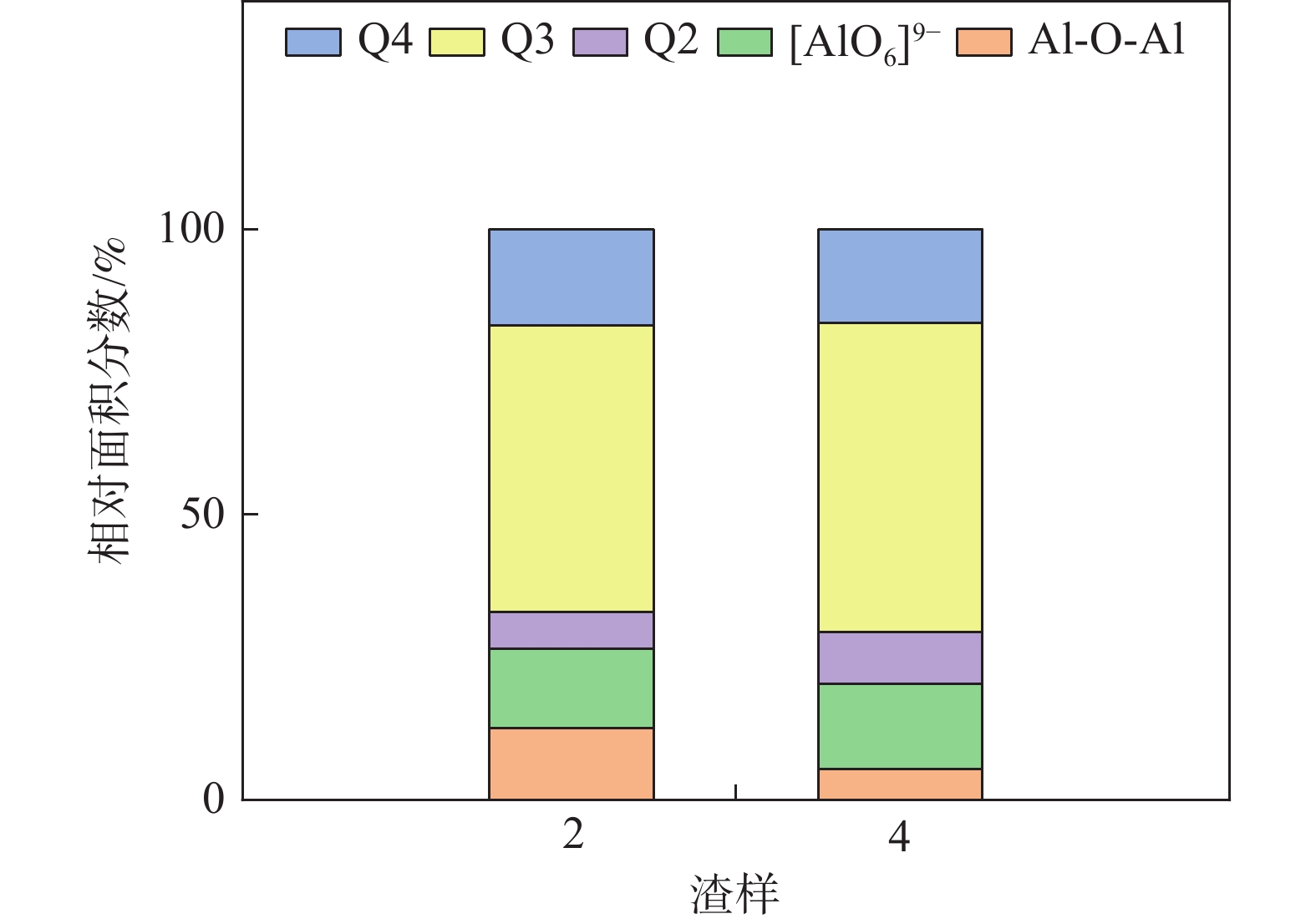
摘要: 采用新型CaO-Al2O3基结晶器保护渣进行高铝钢浇铸,可有效抑制钢液中Al与渣中组元的界面反应。但是,浇铸过程中仍存在着结晶性能强、传热不均等问题。助熔剂是调整熔渣理化性能的关键因素之一。基于此,系统研究了典型助熔剂CaF2对不同酸碱性CaO-Al2O3基保护渣微观结构、黏度及结晶物相的影响。结果表明,在近中性CaO-Al2O3基保护渣中加入CaF2后,析晶物相由Ca12Al14O33+Ca3Al2O6转变为Ca12Al14O32F2+LiAlO2+CaF2,LiAlO2的析出可导致转折点温度上升,升幅约175 ℃,熔渣高温段黏度基本保持不变。在偏碱性CaO-Al2O3基保护渣中加入CaF2后,析晶物相由Ca3Al2O6+CaO转变为Ca12Al14O32F2+LiAlO2+CaF2,单一CaO的过早析出得到有效抑制,转折点温度降低约70 ℃,高温段黏度明显减小。Abstract: The new CaO-Al2O3 based mold fluxes can effectively inhibit the interfacial reaction between Al and slag components in high-aluminum steel casting. However, there were still some problems in the casting process, such as strong crystallization performance and uneven heat transfer. Fluxing agent was one of the key factors to adjust the physical and chemical properties of slag. Based on this, the effects of typical flux CaF2 on the microstructure, viscosity and crystalline phase of CaO-Al2O3 based mold fluxes with different acid-base property were studied. The results show that in the near-neutral CaO-Al2O3-based mold flux, the crystallization phase changes from Ca12Al14O33+Ca3Al2O6 to Ca12Al14O32F2+LiAlO2+CaF2 after adding CaF2. The precipitation of LiAlO2 can lead to the increase of the breaking temperature by about 175 °C, and the viscosity of the high temperature section of the slag remains basically unchanged. In the alkaline CaO-Al2O3-based mold flux, the crystalline phase changed from Ca3Al2O6+CaO to Ca12Al14O32F2+LiAlO2+CaF2 after adding CaF2, and the premature precipitation of single CaO was effectively inhibited. The breaking temperature is reduced by about 70 °C, and the viscosity at high temperature is significantly reduced.
-
Key words:
- continuous casting /
- high-aluminum steel /
- CaO-Al2O3 based mold flux /
- fluxing agent /
- viscosity /
- crystalline phase /
- melt structure
-
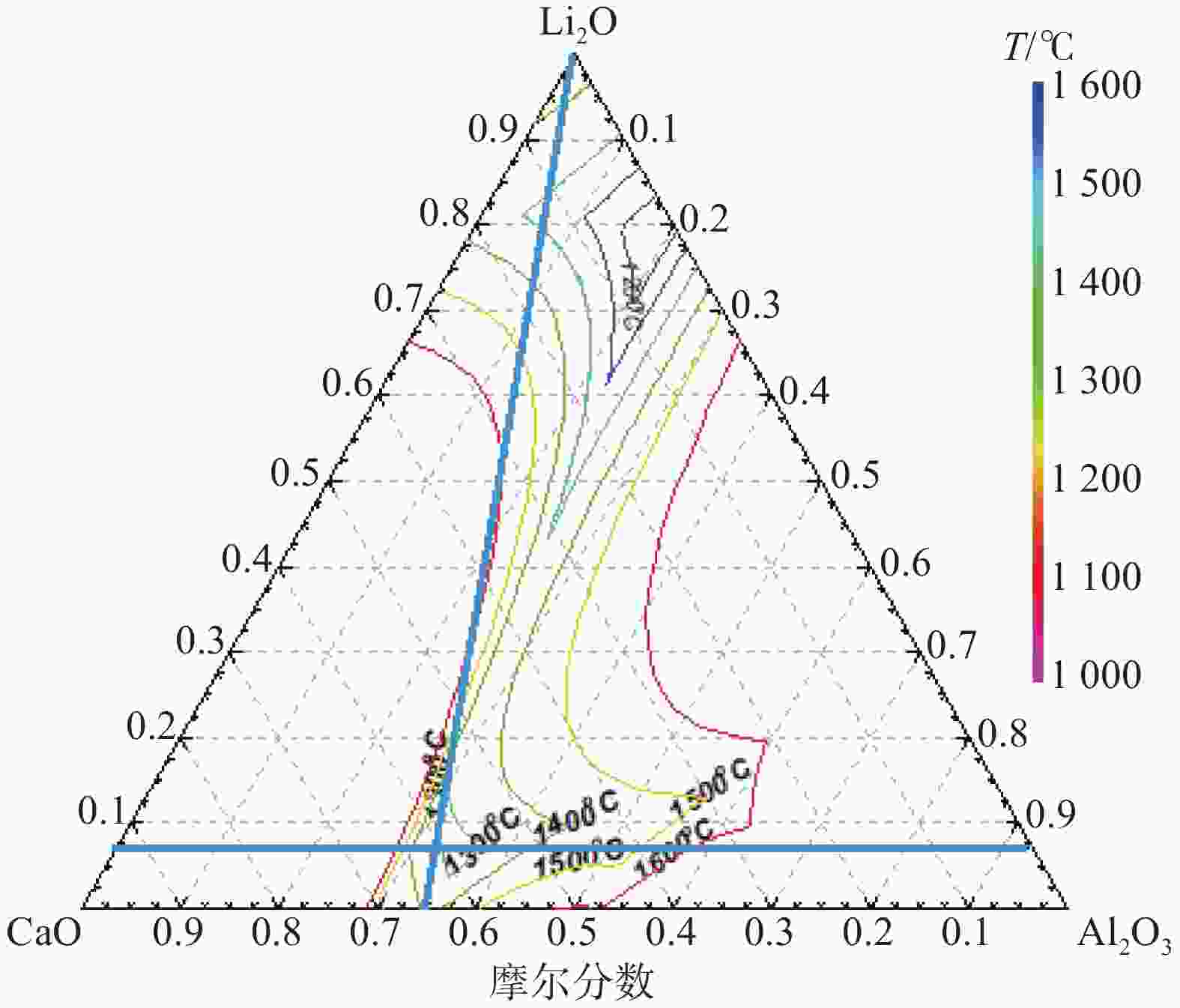
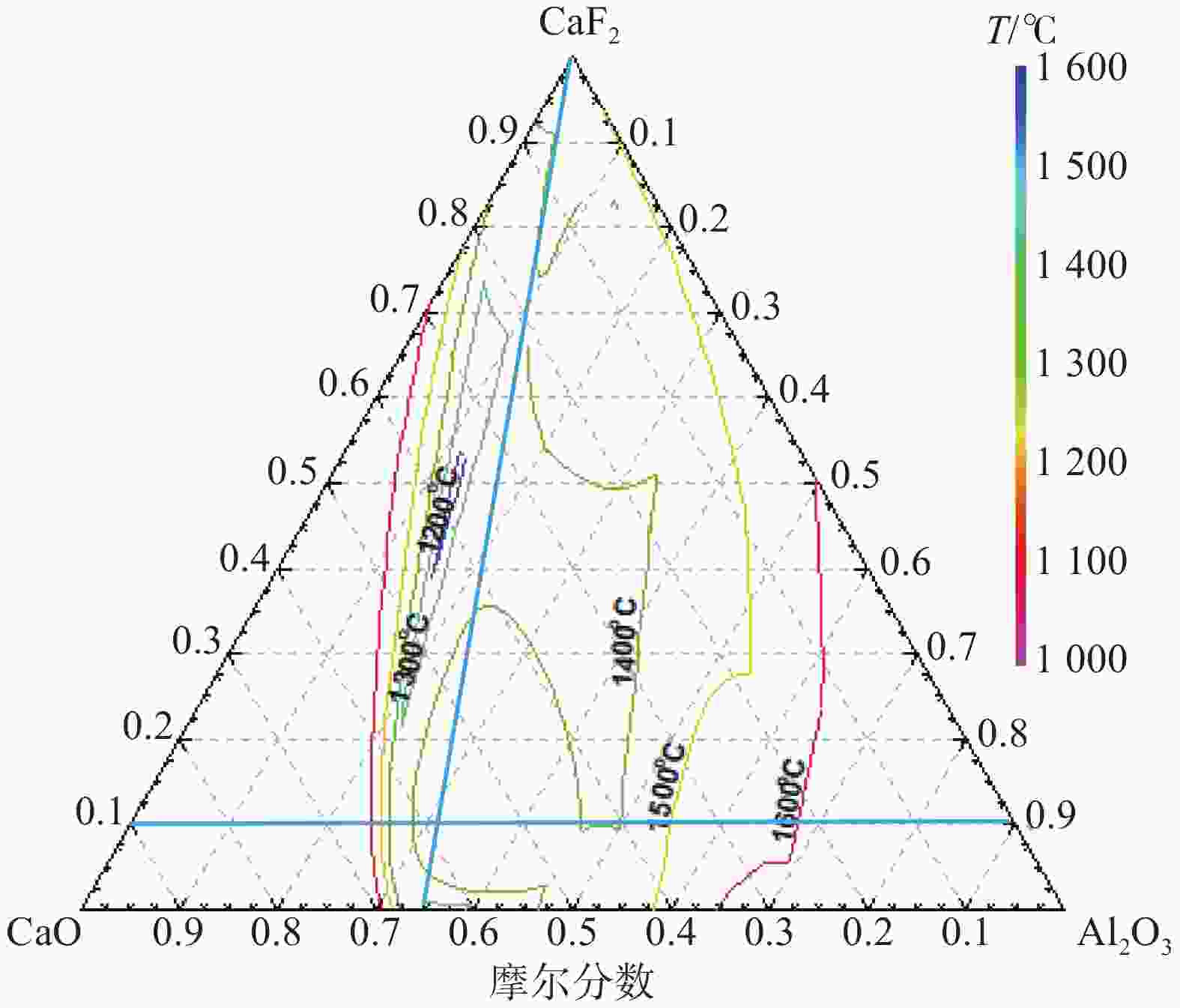
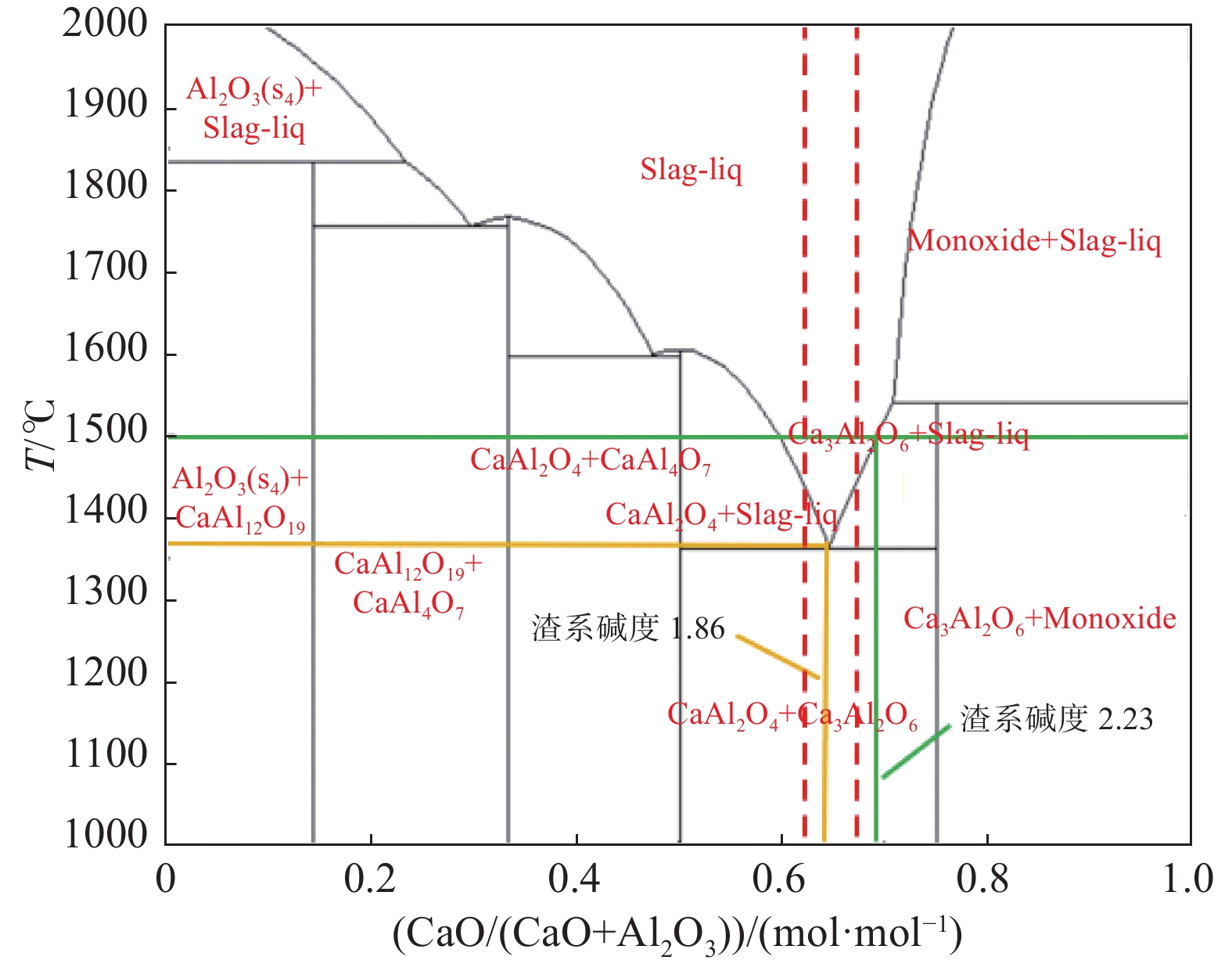
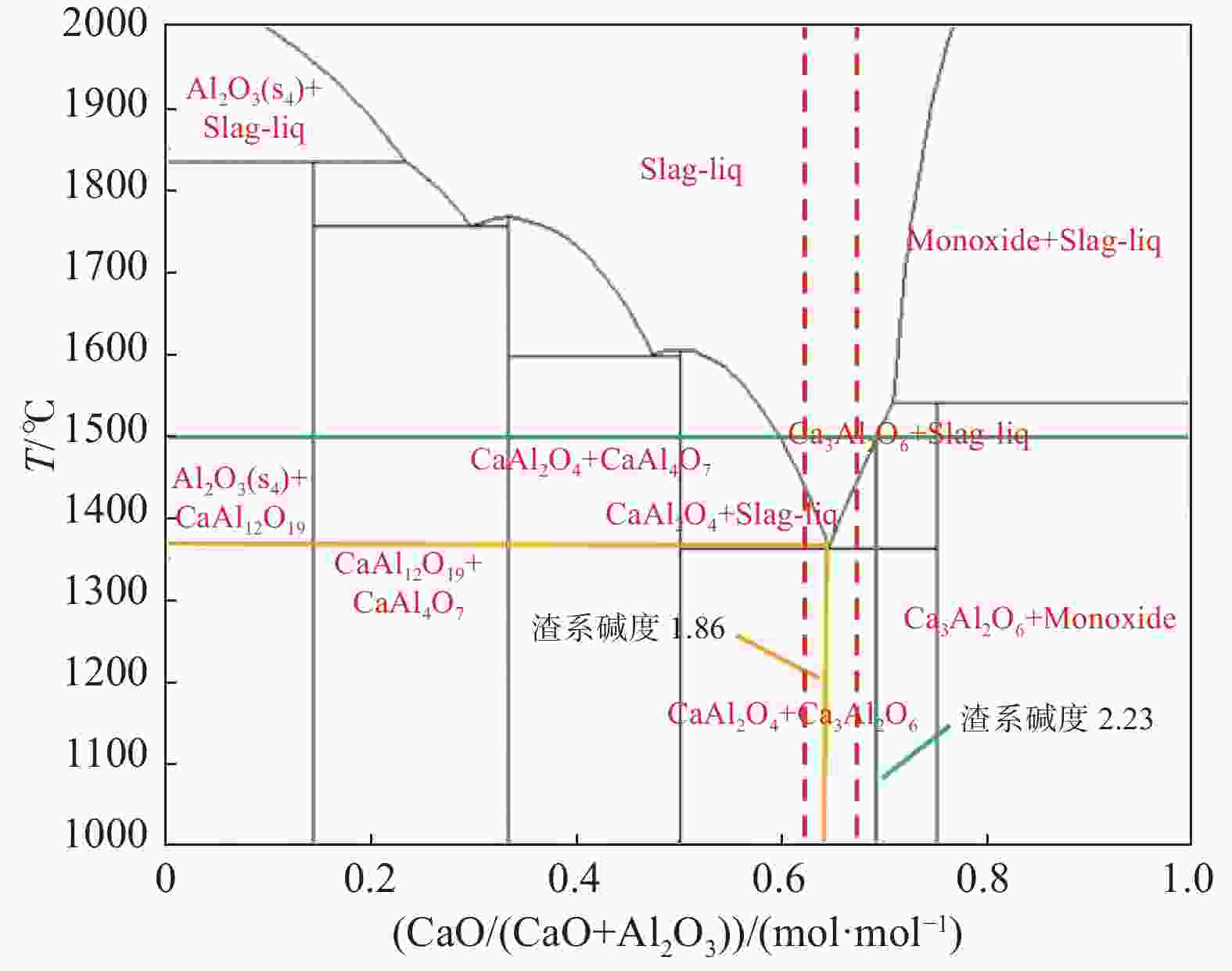
表 1 保护渣的化学成分(摩尔分数)
Table 1. Chemical compositions of mold fluxes (mole fraction)
% 序号 CaO Al2O3 Li2O CaF2 C/A S1 59.8 32.2 8 1.86 S2 63.5 28.5 8 2.23 S3 59.8 32.2 8 10 1.86 S4 63.5 28.5 8 10 2.23 -
[1] Han Wendian, Qiu Shengtao, Zhu Guoling. Development of free-fluorid mold powder[J]. Research on Iron and Steel, 2003,31(2):53−56. (韩文殿, 仇圣桃, 朱果灵. 无氟结晶器保护渣的发展[J]. 钢铁研究, 2003,31(2):53−56. doi: 10.3969/j.issn.1001-1447.2003.02.016Han Wendian, Qiu Shengtao, Zhu Guoling. Development of free-fluorid mold powder[J]. Research on Iron and Steel, 2003, 31(2): 53-56. doi: 10.3969/j.issn.1001-1447.2003.02.016 [2] Song Tushun, Zhu Liguang, Wang Xingjuan, et al. Research of Q345B steel protective slag microstructure and the mineral composition[J]. Continuous Casting, 2017,42(5):29−32. (宋土顺, 朱立光, 王杏娟, 等. Q345B钢保护渣显微结构及矿相组成研究[J]. 连铸, 2017,42(5):29−32.Song Tushun, Zhu Liguang, Wang Xingjuan, et al. Research of Q345 B steel protective slag microstructure and the mineral composition[J]. Continuous Casting, 2017, 42(5): 29-32. [3] Zhu Liguang, Yuan Zhipeng, Xiao Pengcheng, et al. Research and optimization of mold flux for high speed continuous casting of low carbon steel thin slab[J]. Iron and Steel, 2020,55(11):65−73. (朱立光, 袁志鹏, 肖鹏程, 等. 低碳钢薄板坯高速连铸保护渣研究与优化[J]. 钢铁, 2020,55(11):65−73.Zhu Liguang, Yuan Zhipeng, Xiao Pengcheng, et al. Research and optimization of mold flux for high speed continuous casting of low carbon steel thin slab[J]. Iron and Steel, 2020, 55(11): 65-73. [4] Zhang Chen, Cai Dexiang. Investigation on development of low-Li2O mold fluxes used for high aluminum steel[J]. Steelmaking, 2017,33(3):51−55. (张晨, 蔡得祥. 高铝钢用低Li2O保护渣的开发研究[J]. 炼钢, 2017,33(3):51−55.Zhang Chen, Cai Dexiang. Investigation on development of low-Li2O mold fluxes used for high aluminum steel[J]. Steelmaking, 2017, 33(3): 51-55. [5] Yan X B, Yuan H Z, Zhang S D, et al. Effect of interfacial reaction between CaO–BaO–Al2O3-based mold fluxes and high-Mn-high-Al steels on fundamental properties and lubrication of mold flux[J]. Steel Research International, 2020,91(6):1900581-1900589. doi: 10.1002/srin.201900581 [6] 吴婷. 低反应性连铸保护渣熔体的微结构特征及宏观性能研究[D]. 重庆: 重庆大学, 2017.Wu Ting. Study on microstructure and macroproperty of mould fluxes with low reactivity[D]. Chongqing: Chongqing University, 2017. [7] Ai Xingang, Han Dong, Li Shengli, et al. Production practice and prospect of external liquid mold flux in continuous casting[J]. Iron and Steel, 2019,54(8):132−136. (艾新港, 韩东, 李胜利, 等. 外加液态保护渣连铸生产实践与前景展望[J]. 钢铁, 2019,54(8):132−136.Ai Xingang, Han Dong, Li Shengli, et al. Production practice and prospect of external liquid mold flux in continuous casting[J]. Iron and Steel, 2019, 54(8): 132-136. [8] Dong J K, Park J H. Interfacial reaction between CaO-SiO2-MgO-Al2O3 flux and Fe-xMn-yAl (x=10 and 20 mass pct, y=1, 3, and 6 mass pct) steel at 1873 K (1600 °C)[J]. Metallurgical and Materials Transactions B, 2012,43(4):875−886. doi: 10.1007/s11663-012-9667-x [9] Wang W L, Xu H, Zhai B Y, et al. A review of the melt structure and crystallization behavior of non-reactive mold flux for the casting of advanced high-strength steels[J]. Steel Research International, 2022,93(3):2100073. doi: 10.1002/srin.202100073 [10] Sarkar R, Li Z S. Isothermal and non-isothermal crystallization kinetics of mold fluxes used in continuous casting of steel: A review[J]. Steel Research International, 2021,52(3):1357−1378. [11] Mo Rongzheng, Zhang Lifeng, Ren Ying, et al. Review on effect of composition on viscosity of low-reactive mold flux for high-Al steel[J]. Journal of Iron and Steel Research, 2021,33(8):695−708. (莫嵘臻, 张立峰, 任英, 等. 高铝钢用低反应型保护渣成分对其黏度的影响[J]. 钢铁研究学报, 2021,33(8):695−708.Mo Rongzheng, Zhang Lifeng, Ren Ying, et al. Review on effect of composition on viscosity of low-reactive mold flux for high-Al steel[J]. Journal of Iron and Steel Research, 2021, 33(8): 695-708. [12] Zhu Liguang, Zhang Xiaoshi, Wang Xingjuan, et al. Analysis on melting characteristics of CaO-Al2O3 based special flux for high titanium welding wire steel[J]. China Metallurgy, 2020,30(10):9−16. (朱立光, 张晓仕, 王杏娟, 等. 高钛焊丝钢CaO-Al2O3基专用保护渣熔化特性分析[J]. 中国冶金, 2020,30(10):9−16.Zhu Liguang, Zhang Xiaoshi, Wang Xingjuan, et al. Analysis on melting characteristics of CaO-Al2O3 based special flux for high titanium welding wire steel[J]. China Metallurgy, 2020, 30(10): 9-16. [13] Shao H Q, Gao E Z, Wang W L, et al. Effect of fluorine and CaO/Al2O3 mass ratio on the viscosity and structure of CaO–Al2O3-based mold fluxes[J]. Journal of the American Ceramic Society, 2019,102(8):4440−4449. doi: 10.1111/jace.16322 [14] Yang J, Zhang J, Ostrovski O, et al. Effects of fluorine on solidification, viscosity, structure, and heat transfer of CaO-Al2O3-based mold fluxes[J]. Metallurgical and Materials Transactions B, 2019,50(4):1766−1772. doi: 10.1007/s11663-019-01579-z [15] Yu Xiong, Wen Guanghua, Tang Ping, et al. Effect of F− on physico-chemical properties of mold slag used for high-Al steel[J]. Chinese Journal of Process Engineering, 2010,10(6):1153−1157. (于雄, 文光华, 唐萍, 等. F-对高铝钢连铸保护渣理化性能的影响[J]. 过程工程学报, 2010,10(6):1153−1157.Yu Xiong, Wen Guanghua, Tang Ping, et al. Effect of F− on physico-chemical properties of mold slag used for high-Al steel[J]. Chinese Journal of Process Engineering, 2010, 10(6): 1153-1157. [16] Wang Zhe, Tang Ping, Mi Xiaoxi, et al. Effect of w(CaF2) on crystallization properties of CaO-SiO2-Al2O3 based mold fluxes[J]. Iron and Steel, 2018,53(7):38−44. (王哲, 唐萍, 米晓希, 等. CaF2对CaO-SiO2-Al2O3渣系保护渣结晶行为的影响[J]. 钢铁, 2018,53(7):38−44.Wang Zhe, Tang Ping, Mi Xiaoxi, et al. Effect of w(CaF2) on crystallization properties of CaO-SiO2-Al2O3 based mold fluxes[J]. Iron and Steel, 2018, 53(7): 38-44. [17] 林超. 石煤改质转炉钢渣自粉化及提钒的基础研究[D]. 马鞍山: 安徽工业大学, 2018.Lin Chao. Study on pulverization and vanadium extraction of stone coal modified converter steel slag[D]. Ma, anshan: Anhui University of Technology, 2018. [18] 郭汉杰. 冶金物理化学教程[M]. 北京: 冶金工业出版社, 2004.Guo Hanjie. Tutorial of metallurgical physical chemistry[M]. Beijing: Metallurgical Industry Press, 2004. [19] Mills K C, Sridhar S. Viscosities of ironmaking and steelmaking slags[J]. Ironmaking and Steelmaking, 1999,26(4):262−268. doi: 10.1179/030192399677121 [20] Schulz T, Lychatz B, Haustein N, et al. Structurally based assessment of the influence of fluorides on the characteristics of continuous casting powder slags[J]. MMTB, 2013,44(2):317−327. doi: 10.1007/s11663-013-9796-x [21] Li Z, You X, Li M, et al. Effect of substituting CaO with BaO and CaO/Al2O3 ratio on the viscosity of CaO–BaO–Al2O3–CaF2–Li2O mold flux system[J]. Metals, 2019,9(2):142. doi: 10.3390/met9020142 [22] Shankar A, Görnerup M, Lahiri A. Estimation of viscosity for blast furnace type slags[J]. Ironmaking and Steelmaking, 2007,34:477−481. doi: 10.1179/174328107X17467 [23] Li Chen, Qi Jie, Liu Chengjun, et al. Effect of fluxing agent on the properties of CaO-Al2O3 based mold flux[J]. Iron Steel Vanadium Titanium, 2021,42(4):124−130. (李晨, 亓捷, 刘承军, 等. 助熔剂对CaO-Al2O3基保护渣理化性能的影响[J]. 钢铁钒钛, 2021,42(4):124−130. doi: 10.7513/j.issn.1004-7638.2021.04.021Li Chen, Qi Jie, Liu Chengjun, et al. Effect of fluxing agent on the properties of CaO-Al2O3 based mold flux[J]. Iron Steel Vanadium Titanium, 2021, 42(4): 124-130. doi: 10.7513/j.issn.1004-7638.2021.04.021 [24] Park J H, Min D J, Song H S. Structural investigation of CaO–Al2O3 and CaO–Al2O3–CaF2 slags via fourier transform infrared spectra[J]. ISIJ International, 2002,42(1):38−43. doi: 10.2355/isijinternational.42.38 [25] Hyun K G, Sohn I. Effect of CaF2, B2O3 and the CaO/SiO2 mass ratio on the viscosity and structure of B2O3 ontaining calcium -ilicate-based melts[J]. Journal of the American Ceramic Society, 2019,102(11):6575−6590. doi: 10.1111/jace.16526 [26] Porto S, Krishnan R S. Raman effect of corundum[J]. Journal of Chemical Physics, 1967,47(3):1009−1012. doi: 10.1063/1.1711980 [27] Tarte P. Infra-red spectra of inorganic aluminates and characteristic vibrational frequencies of AlO4 tetrahedra and AlO6 octahedra[J]. Spectrochimica Acta Part A:Molecular Spectroscopy, 1967,23(7):2127−2143. doi: 10.1016/0584-8539(67)80100-4 [28] Kiss A B, Keresztury G, Farkas L. Raman and ir spectra and structure of boehmite (γ-AlOOH). Evidence for the recently discarded D172h space group[J]. Spectrochimica Acta Part A:Molecular Spectroscopy, 1980,36(7):653−658. doi: 10.1016/0584-8539(80)80024-9 [29] Wang Y, Zhang R, Zhao X, et al. Structural transformation of molten CaO–SiO2–Al2O3–FexO slags during secondary refining of steels[J]. ISIJ International, 2020,60(2):220−225. doi: 10.2355/isijinternational.ISIJINT-2019-418 [30] Kim T S, Park J H. Structure-viscosity relationship of low-silica calcium aluminosilicate melts[J]. ISIJ International, 2014,54(9):2031−2038. doi: 10.2355/isijinternational.54.2031 [31] Gao E Z, Wang W L, Zhang L. Effect of alkaline earth metal oxides on the viscosity and structure of the CaO-Al2O3 based mold flux for casting high-al steels[J]. Journal of Non-crystalline Solids, 2017,473:79−86. doi: 10.1016/j.jnoncrysol.2017.07.029 [32] Kim G H, Sohn I. Effect of Al2O3 on the viscosity and structure of calcium silicate-based melts containing Na2O and CaF2[J]. Journal of Non-crystalline Solids, 2012,358(12-13):1530−1537. doi: 10.1016/j.jnoncrysol.2012.04.009 [33] Zhang X B, Liu C J, Jiang M F. Effect of fluorine on melt structure for CaO-SiO2-CaF2 and CaO-Al2O3-CaF2 by molecular dynamics simulations[J]. ISIJ International, 2020,60(10):2176−2182. doi: 10.2355/isijinternational.ISIJINT-2020-002 [34] Wang X J, Jin H, Zhu L G, et al. Effect of CaF2 on the viscosity and microstructure of CaO–SiO2–Al2O3 based continuous casting mold flux[J]. Metals, 2019,9(8):871. doi: 10.3390/met9080871 -



 下载:
下载: