Research on preparation of metal vanadium by carbothermal reduction
-
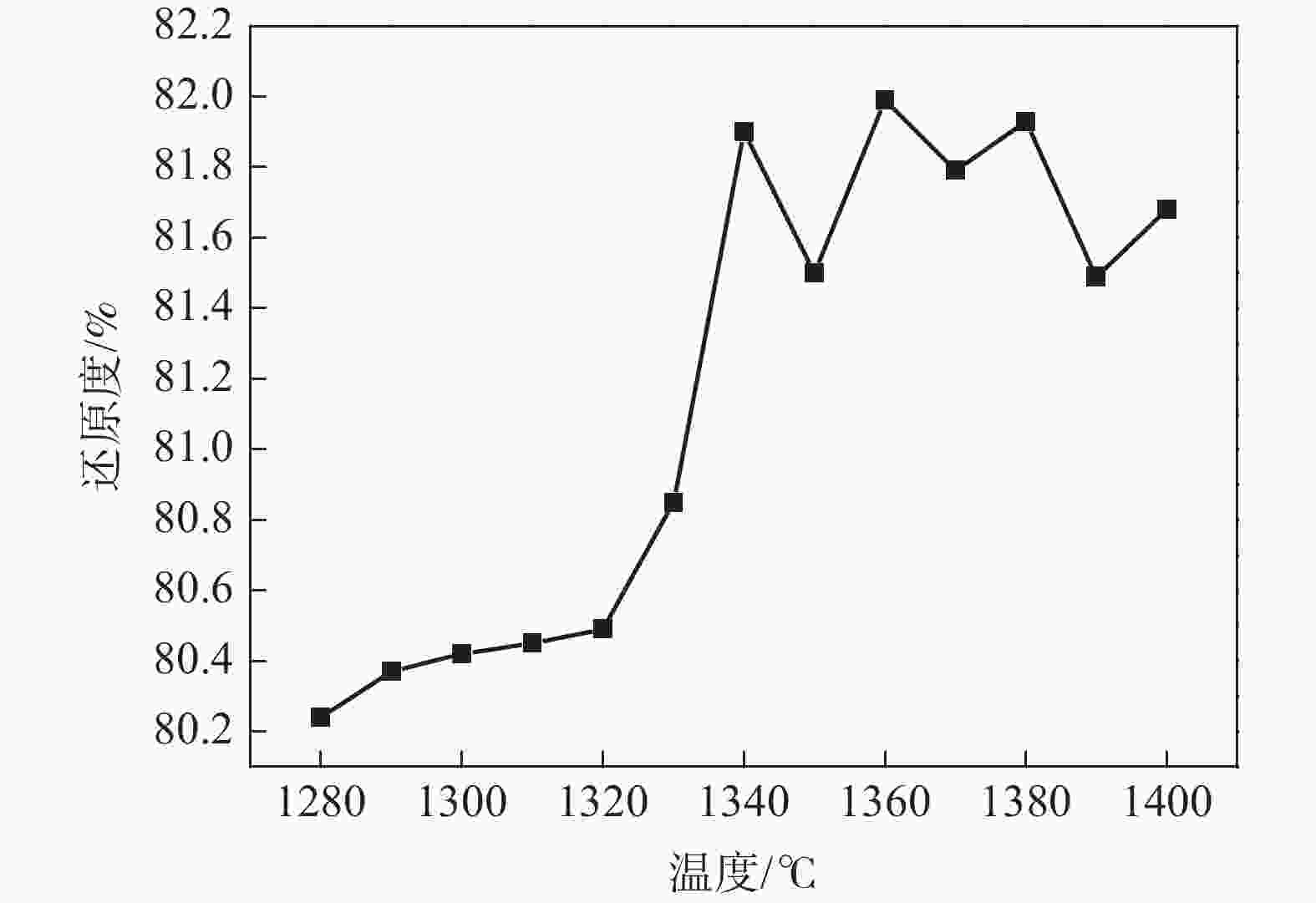
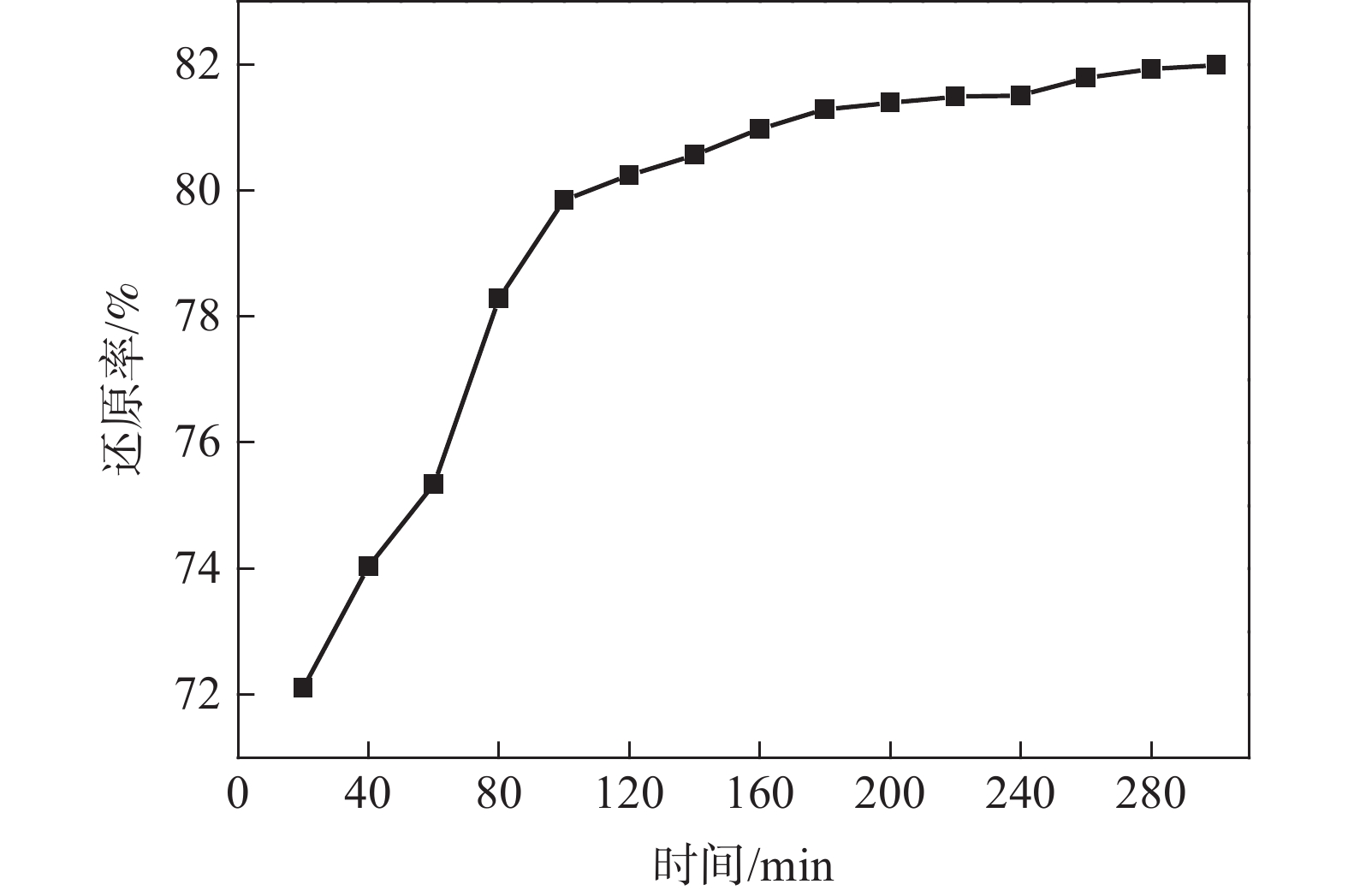
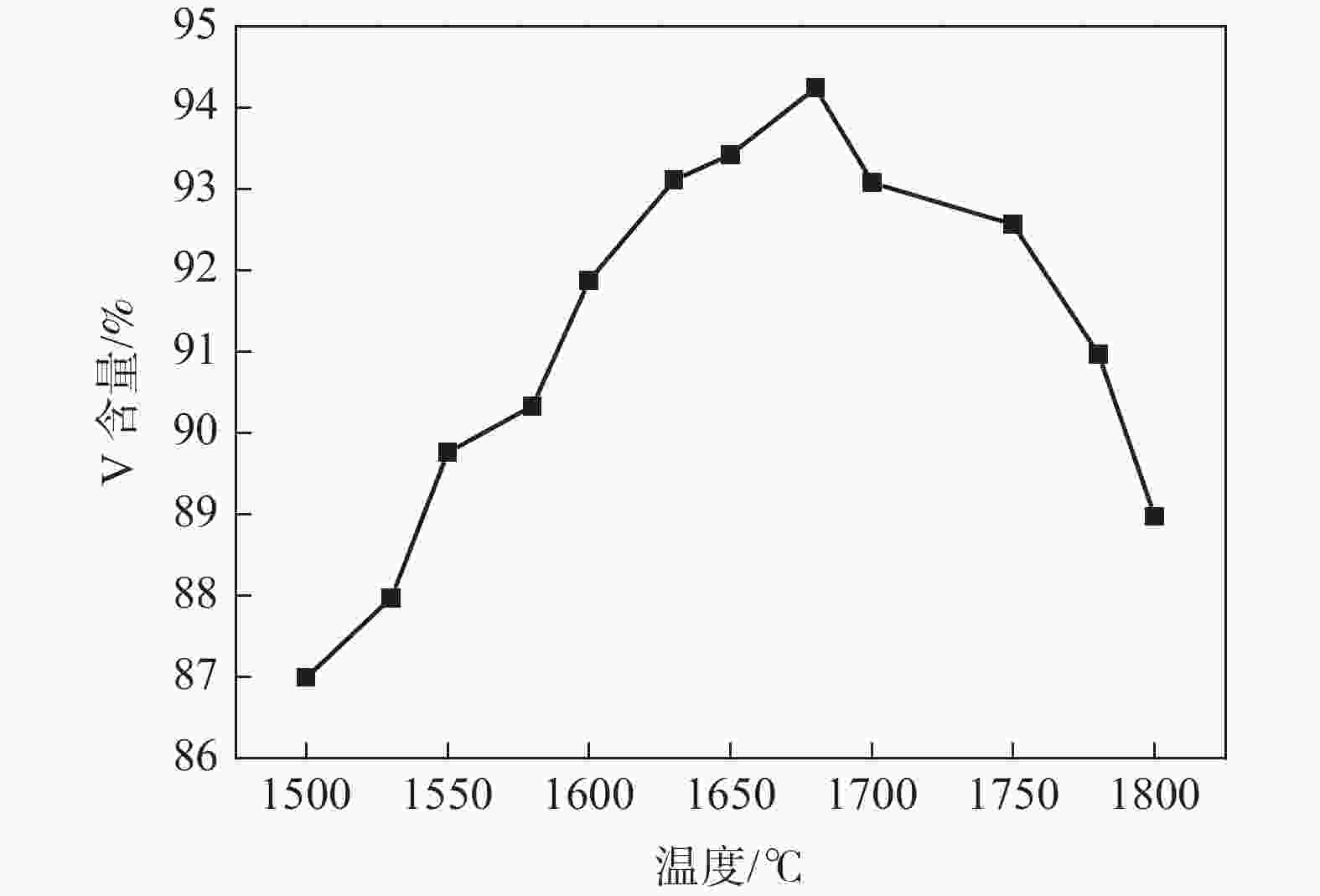
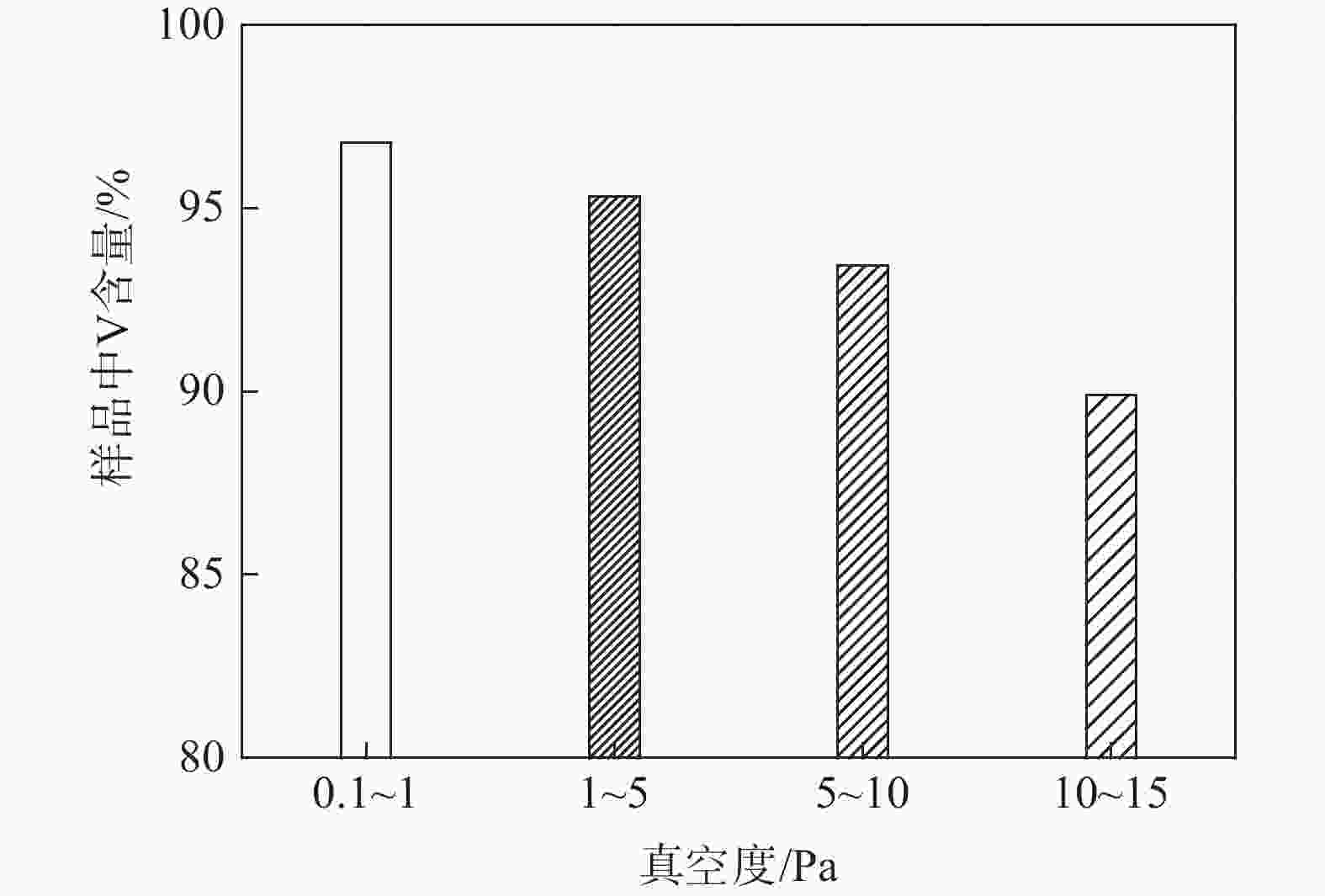
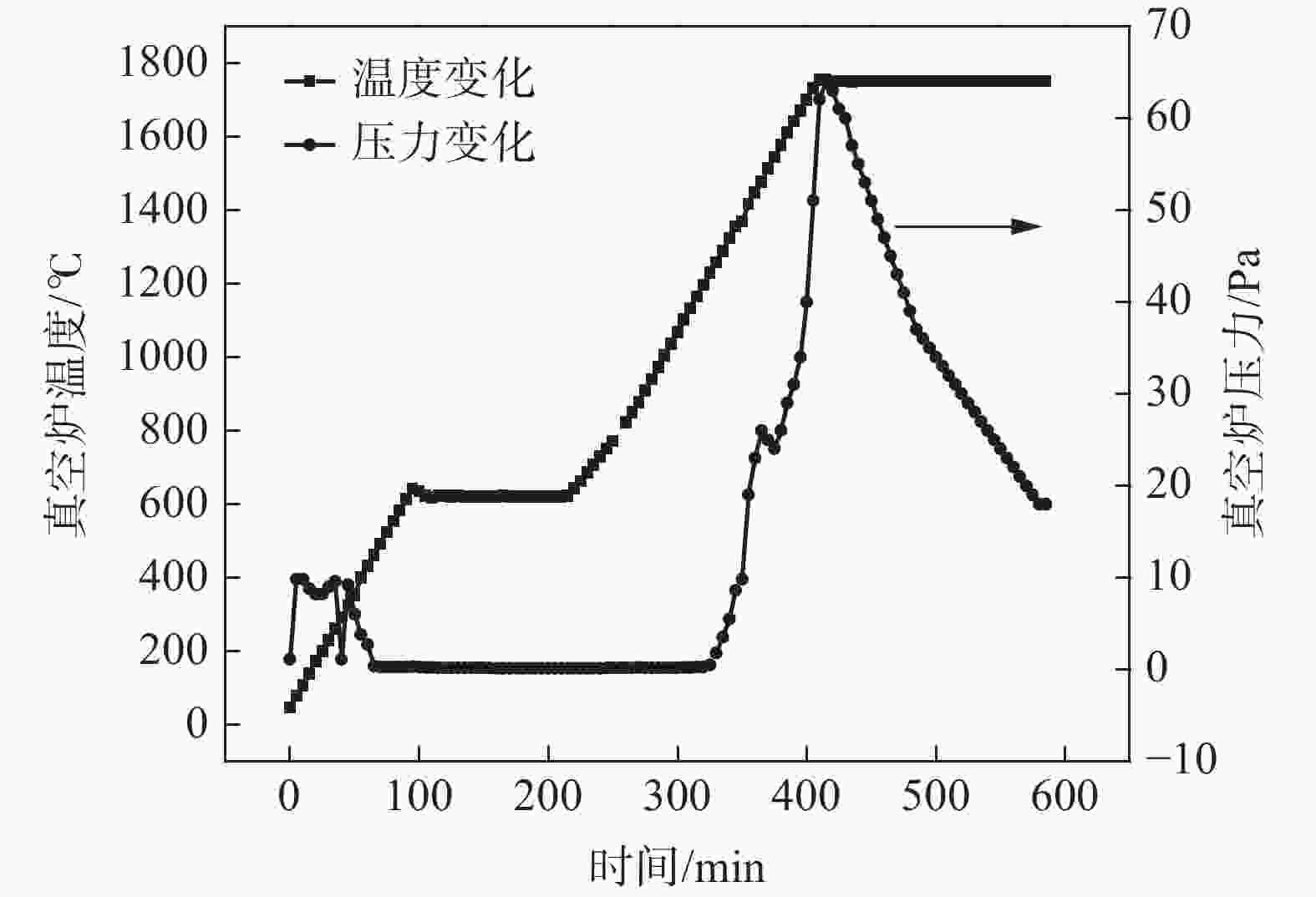
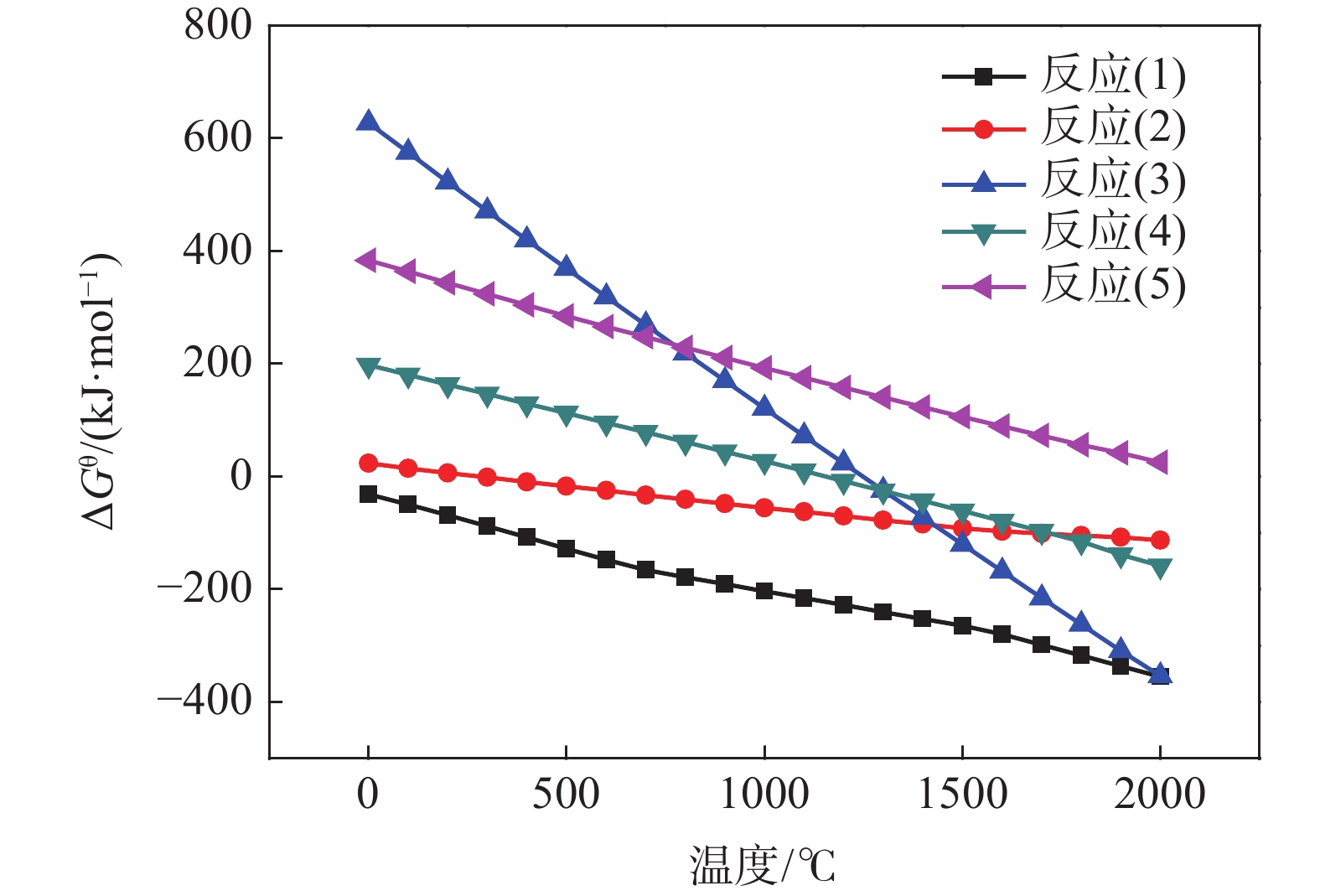
摘要: 以高纯V2O5粉末为原料,C粉末为还原剂,对碳热还原制备金属钒过程进行研究,重点讨论了配碳量、温度、真空度和还原时间对钒中间体和精炼提纯过程的影响。结果表明,V2O5为逐级还原,存在直接和间接还原。配碳比32%,还原温度控制在1350 ℃,保温时间为120 min时,得到的粗钒固溶体相以<VO,V2C>为主,钒含量为84%左右。精炼提纯条件为温度1680 ℃,碳氧比1.02,真空度在0.1 Pa以下时,能得到延展性金属钒产品,纯度达到99.04%。Abstract: High-purity V2O5 powder was used as raw material and carbon powder was used as reducing agent. The process of preparing metals by carbothermal reduction was studied, and the effects of the amount of carbon, temperature, vacuum degree and reduction time on the products were discussed. The results show that V2O5 is a stepwise reduction with direct and indirect reduction. With a carbon ratio of 32%, the reduction temperature at 1350 ℃, and the reduction time of 120 min, the obtained crude vanadium solid solution phase is mainly <VO, V2C>, and the V content is about 84%. For refining conditions, when the temperature is controlled at 1680 ℃, the carbon-oxygen ratio is 1.02, and the vacuum degree is below 0.1 Pa, the ductile metal vanadium product can be obtained, and the purity of vanadium reaches 99.04%.
-
Key words:
- metal vanadium /
- V2O5 /
- carbothermal reduction /
- crude vanadium /
- purification
-
表 1 产品成分ICP分析
Table 1. ICP analysis of product composition
% V C O Al Ca Cr Fe Mg Mn Si 99.04 0.231 0.098 0.0787 0.161 0.005 0.1106 0.0141 0.009 0.0794 -
[1] Liu Shuqing. Production status and development trend of vanadium product in world in recent years[J]. Iron Steel Vanadium Titanium, 2014,35(3):55−62. (刘淑清. 近年全球钒制品生产现状及发展趋势[J]. 钢铁钒钛, 2014,35(3):55−62. doi: 10.7513/j.issn.1004-7638.2014.03.013Liu Shuqing. Production status and development trend of vanadium product in world in recent years[J]. Iron Steel Vanadium Titanium, 2014, 35(3): 55−62. doi: 10.7513/j.issn.1004-7638.2014.03.013 [2] Xian Xiaobin, Ye Linsen, Leng Bangyi, et al. Study on preparation and properties of pure vanadium[J]. Rare Metal Materials and Engineering, 2010,39(8):928−931. (鲜晓斌, 叶林森, 冷邦义, 等. 纯钒制备及其性能[J]. 稀有金属材料与工程, 2010,39(8):928−931.Xian Xiaobin, Ye Linsen, Leng Bangyi, et al. Study on preparation and properties of pure vanadium[J]. Rare Metal Materials and Engineering, 2010, 39(8): 928−931. [3] Fan Liang, Zhang Wei. Vanadium resource and its preparation technology[J]. Advanced Materials Industry, 2016(1):41−46. (范亮, 张炜. 钒资源及其制备技术[J]. 新材料产业, 2016(1):41−46. doi: 10.3969/j.issn.1008-892X.2016.01.010Fan Liang, Zhang Wei. Vanadium resource and its preparation technology[J]. Advanced Materials Industry, 2016(1): 41−46. doi: 10.3969/j.issn.1008-892X.2016.01.010 [4] Liao Shiming, Bai Tanlun. Vanadium metallurgy abroad[M]. Beijing: Metallurgical Industry Press, 1985. (廖世明, 柏谈论. 国外钒冶金[M]. 北京: 冶金工业出版社, 1985.Liao Shiming, Bai Tanlun. Vanadium metallurgy abroad[M]. Beijing: Metallurgical Industry Press, 1985. [5] Hou Shuai, Tian Ying, Li Yungang. Research progress in preparation methods of vanadium metal[J]. Rare Metals and Cemeted Carbides, 2022,50(6):22−26, 32. (侯帅, 田颖, 李运刚. 金属钒制备方法的研究进展[J]. 稀有金属与硬质合金, 2022,50(6):22−26, 32.Hou Shuai, Tian Ying, Li Yungang. Research progress in preparation methods of vanadium metal[J]. Rare Metals and Cemeted Carbides, 2022, 50(6): 22−26, 32. [6] Lei K P V, Campbell R E, Sullivan T A. Electrolytic preparation of vanadium from vanadium carbide[J]. Journal of Electrochemical Society, 1973,120(2):211−215. doi: 10.1149/1.2403422 [7] Xiong Weihua, Wu Jun, Zhang Zhenhua,et al. Domestic development of vanadium detector for core self-sufficient energy in Qinshan No. 3 nuclear power plant[C]// Proceedings of the 7th National Conference on Nuckar Instrument and Its Application & the 5th Nuclear Reactou Instrument Conference. Xining: Chinese Institute of Electronics, Chinese Nuclear Society, 2009. (熊伟华, 吴军, 张振华, 等. 秦山第三核电厂堆芯自给能钒探测器国产化研制[C]. 第七届全国核仪器及其应用学术会议暨全国第五届核反应堆用核仪器学术会议论文集. 西宁: 中国电子学会, 中国核学会, 2009.Xiong Weihua, Wu Jun, Zhang Zhenhua,et al. Domestic development of vanadium detector for core self-sufficient energy in Qinshan No. 3 nuclear power plant[C]// Proceedings of the 7th National Conference on Nuckar Instrument and Its Application & the 5th Nuclear Reactou Instrument Conference. Xining: Chinese Institute of Electronics, Chinese Nuclear Society, 2009. [8] Moskalyk R R, Alfantazi A M. Processing of vanadium: a review[J]. Minerals Engineering, 2003,16(9):793−805. doi: 10.1016/S0892-6875(03)00213-9 [9] Wang T, Xu L, Liu C, et al. Calcified roasting-acid leaching process of vanadium from low-grade vanadium-containing stone coal[J]. Chinese Journal of Geochemistry, 2014,33(2):163−167. doi: 10.1007/s11631-014-0672-4 [10] Sun Zhaohui. Understanding the situation clearly promoting sustainable development on China’s vanadium industry[J]. Ferro-alloys, 2008(6):44−48. (孙朝晖. 充分认清形势, 促进中国钒产业的可持续发展[J]. 铁合金, 2008(6):44−48. doi: 10.3969/j.issn.1001-1943.2008.06.011Sun Zhaohui. Understanding the situation clearly promoting sustainable development on China’s vanadium industry[J]. Ferro-alloys, 2008(6): 44−48. doi: 10.3969/j.issn.1001-1943.2008.06.011 [11] Jon Hykawy. Vanadium supercharger[R]. Byron Capital Market, 2009, November. [12] Ulaganathan M, Arabindan V, Yan Q Y, et al. Recent advancements in all-vanadium redox flow batteries[J]. Advanced Materials Interfaces, 2016,3(1):1−22. [13] Kumar S, Jain A, Ichikawa T, et al. Development of vanadium based hydrogen storage material: a review[J]. Renewable and Sustainable Energy Reviews, 2017,72:791−800. doi: 10.1016/j.rser.2017.01.063 [14] Koyama K, Hashimoyo Y, Omori S, et al. Carbothermic reduction of V2O3 under reduced pressure[J]. Transactions of the Japan Institute of Metals, 1982,23(8):451−460. doi: 10.2320/matertrans1960.23.451 [15] Ono K, Moriyama J. Carbothermic reduction and electron beam melting of vanadium[J]. Journal of the Less Common Metals, 1981,81:79−89. doi: 10.1016/0022-5088(81)90271-X [16] Anonymous. Preparation of plastic vanadium by carbon reduction metal vanadium block[J]. Rare Metal Materials and Engineering, 1974(3):25−40. (佚名. 用碳还原金属钒块制取可塑性钒的研制[J]. 稀有金属合金加工, 1974(3):25−40.Anonymous. Preparation of plastic vanadium by carbon reduction metal vanadium block[J]. Rare Metal Materials and Engineering, 1974(3): 25−40. [17] Wang Yanhui, Liu Qi, Bo Xinwei, et al. Research on sintering performance of high purity metal vanadium powders[J]. Powder Metallurgy Technology, 2019,37(5):339−343, 349. (王焱辉, 刘奇, 薄新维, 等. 高纯金属钒粉烧结性能研究[J]. 粉末冶金技术, 2019,37(5):339−343, 349.Wang Yanhui, Liu Qi, Bo Xinwei, et al. Research on sintering performance of high purity metal vanadium powders[J]. Powder Metallurgy Technology, 2019, 37(5): 339−343, 349. -





 下载:
下载: