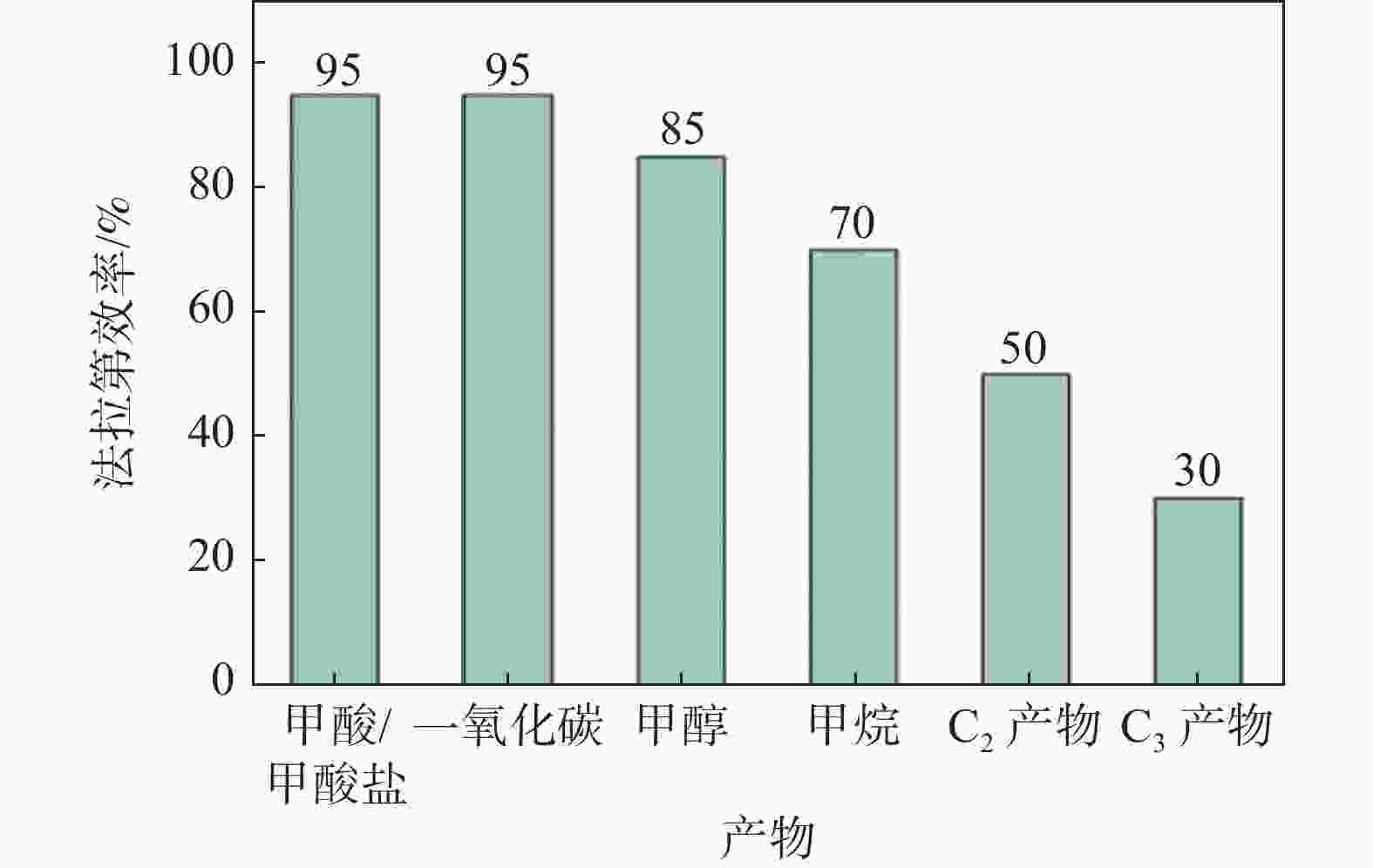
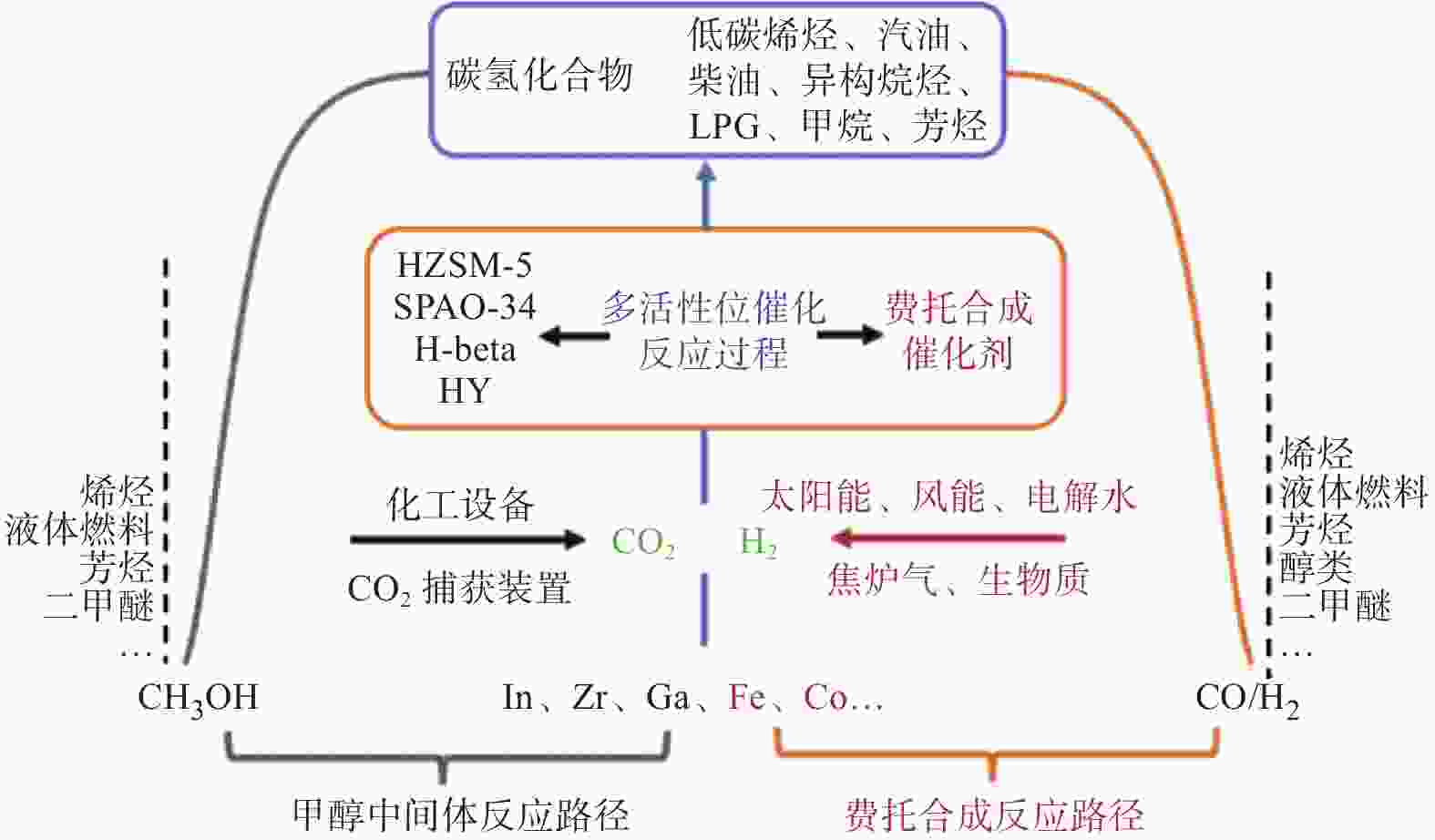
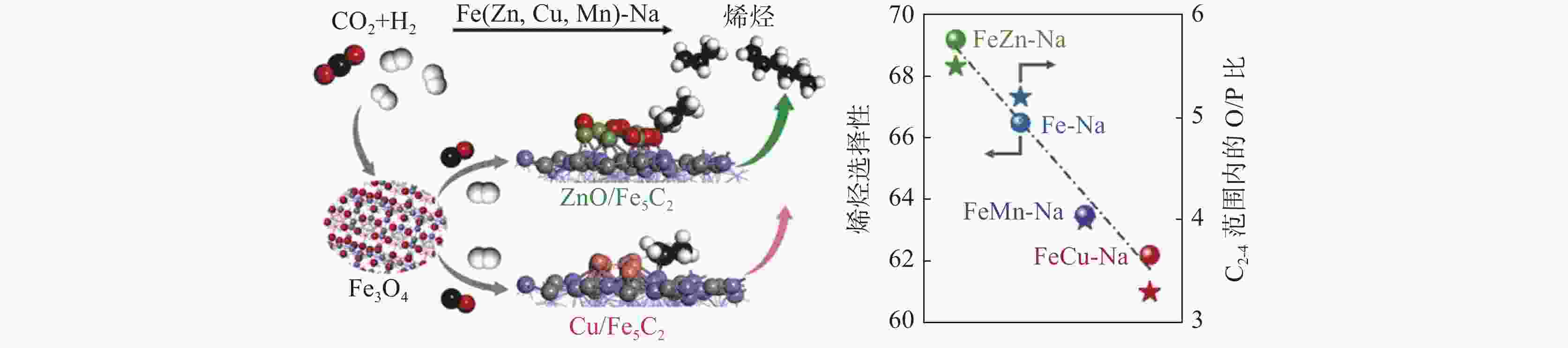
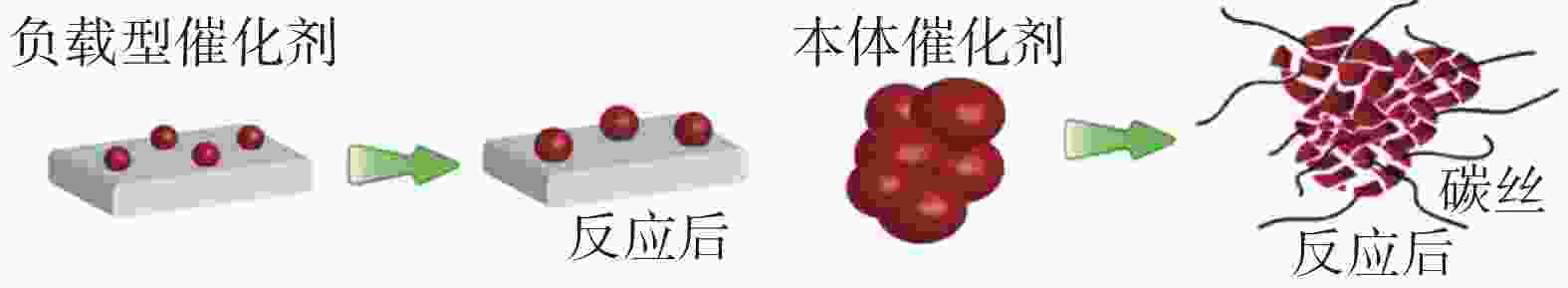
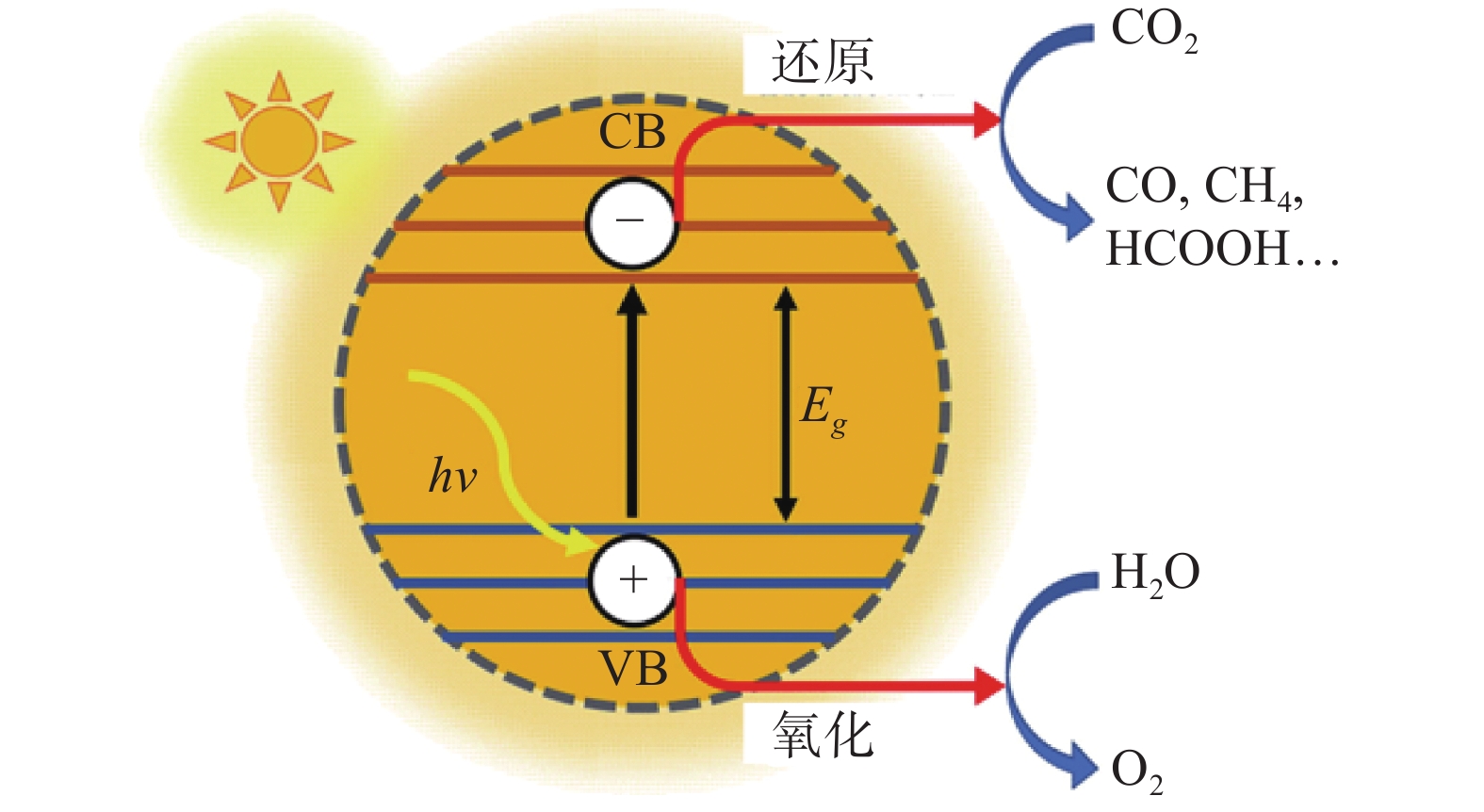
| [1] |
IEA. IEA (2024), CO2 Emissions in 2023[Z/OL]. https://www.iea.org/reports/co2-emissions-in-2023.
|
| [2] |
ZHOU Z X, GAO P. Direct carbon dioxide hydrogenation to produce bulk chemicals and liquid fuels via heterogeneous catalysis[J]. Chinese Journal of Catalysis, 2022, 43(8): 2045-2056. doi: 10.1016/S1872-2067(22)64107-X
|
| [3] |
LI C X, ZHANG Y, ZHANG K X, et al. Research on resource utilization of CO2 in steel industry[J]. Materials Reports, 2023, 37(S2): 468-473. (李晨晓, 张昀, 张凯璇, 等. 钢铁行业中CO2资源化利用的研究进展[J]. 材料导报, 2023, 37(S2): 468-473.LI C X, ZHANG Y, ZHANG K X, et al. Research on resource utilization of CO2 in steel industry[J]. Materials Reports, 2023, 37(S2): 468-473.
|
| [4] |
ÁLVAREZ A, BANSODE A, URAKAWA A, et al. Challenges in the greener production of formates/formic acid, methanol, and DME by heterogeneously catalyzed CO2 hydrogenation processes[J]. Chemical reviews, 2017, 117(14): 9804-9838. doi: 10.1021/acs.chemrev.6b00816
|
| [5] |
RODRIGUEZ J A, EVANS J, FERIA L, et al. CO2 hydrogenation on Au/TiC, Cu/TiC, and Ni/TiC catalysts: Production of CO, methanol, and methane[J]. Journal of catalysis, 2013, 307: 162-169. doi: 10.1016/j.jcat.2013.07.023
|
| [6] |
WANG F, HARINDINTWALI J D, YUAN Z Z, et al. Technologies and perspectives for achieving carbon neutrality[J]. The innovation, 2021, 2(4):100180.
|
| [7] |
LIU J Y, LI P S, JIA S Q, et al. Electrocatalytic CO2 hydrogenation to C2+ alcohols catalysed by Pr–Cu oxide heterointerfaces[J]. Nature Synthesis, 2025: 1-14.
|
| [8] |
ONO S, KANEGA R, KAWANAMI H. Direct formic acid production by CO2 hydrogenation with Ir complexes in HFIP under supercritical conditions[J]. Organometallics, 2024, 43(19): 2213-2220. doi: 10.1021/acs.organomet.4c00229
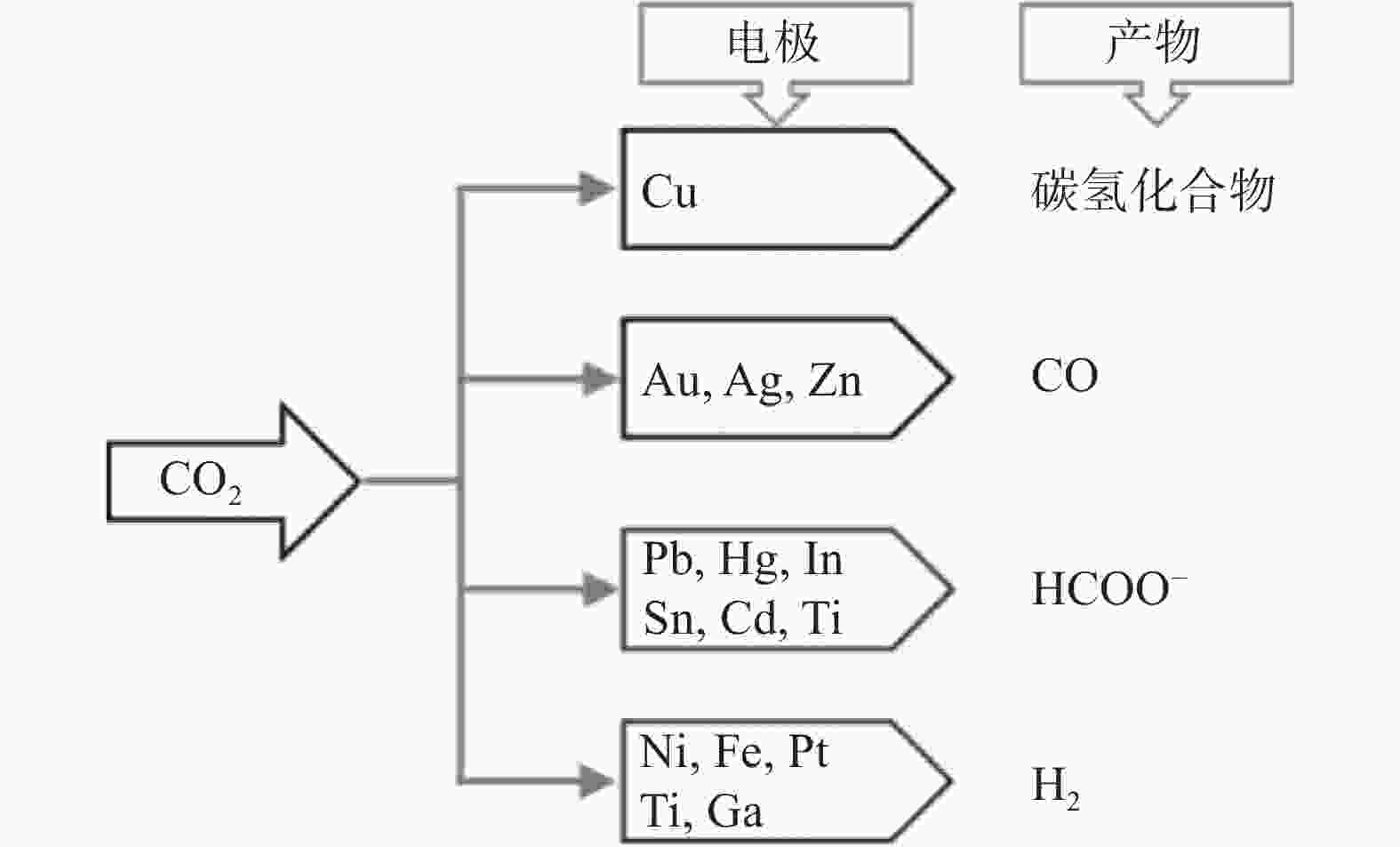
|
| [9] |
RONDA-LLORET M, ROTHENBERG G, SHIJU N R. A critical look at direct catalytic hydrogenation of carbon dioxide to olefins[J]. ChemSusChem, 2019, 12(17): 3896-3914. doi: 10.1002/cssc.201900915
|
| [10] |
ZHAO J B, BIAN F M. Progress on basis and application of CO2 chemical conversion technologies[J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 524-535. (赵锦波, 卞凤鸣. CO2化学转化基础与应用研究进展[J]. 化工进展, 2022, 41(S1): 524-535.ZHAO J B, BIAN F M. Progress on basis and application of CO2 chemical conversion technologies[J]. Chemical Industry and Engineering Progress, 2022, 41(S1): 524-535.
|
| [11] |
NANDAL N, JAIN S L. A review on progress and perspective of molecular catalysis in photoelectrochemical reduction of CO2[J]. Coordination Chemistry Reviews, 2022, 451: 214271. doi: 10.1016/j.ccr.2021.214271
|
| [12] |
WU H, MENG F M. Research progress of modified nano-TiO2 composite materials in the field of photocatalysis[J]. Material Sciences, 2021, 11: 1003. (吴昊, 孟凡明. 改性纳米TiO2复合材料在光催化领域研究进展[J]. 材料科学, 2021, 11: 1003. doi: 10.12677/MS.2021.119116WU H, MENG F M. Research progress of modified nano-TiO2 composite materials in the field of photocatalysis[J]. Material Sciences, 2021, 11: 1003. doi: 10.12677/MS.2021.119116
|
| [13] |
ZHANG X, ZHANG Z Q, SUN Y D, et al. A review on WO3-based composite photocatalysts: synthesis, catalytic mechanism and diversified applications[J]. Rare Metals, 2024: 1-19.
|
| [14] |
QIAN Y, ZHANG F, PANG H. A review of MOFs and their composites-based photocatalysts: synthesis and applications[J]. Advanced Functional Materials, 2021, 31(37): 2104231. doi: 10.1002/adfm.202104231
|
| [15] |
ZHAO B B, ZHONG W, CHEN F, et al. High-crystalline g-C3N4 photocatalysts: Synthesis, structure modulation, and H2-evolution application[J]. Chinese Journal of Catalysis, 2023, 52(9): 127. (赵彬彬, 钟威, 陈峰, 等. 高晶化 g-C3N4 光催化剂: 合成, 结构调控和光催化产氢[J]. 催化学报, 2023, 52(9): 127.ZHAO B B, ZHONG W, CHEN F, et al. High-crystalline g-C3N4 photocatalysts: Synthesis, structure modulation, and H2-evolution application[J]. Chinese Journal of Catalysis, 2023, 52(9): 127.
|
| [16] |
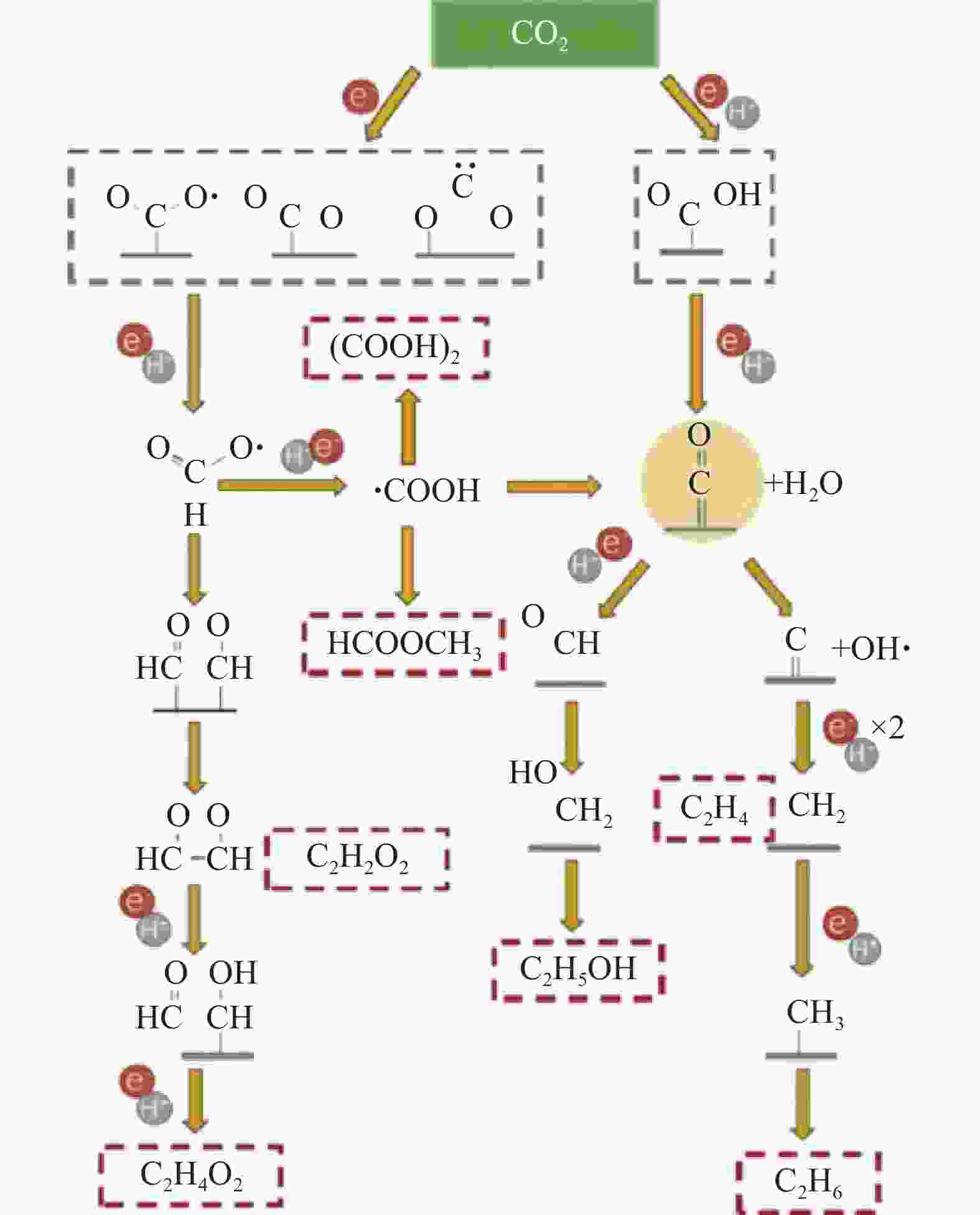
NING S, OU H, LI Y, et al. Co0- Coδ+ Interface double-site-mediated C−C coupling for the photothermal conversion of CO2 into light olefins[J]. Angewandte Chemie International Edition, 2023, 62(23): e202302253. doi: 10.1002/anie.202302253
|
| [17] |
DU C Y, SHENG J P, ZHONG F Y, et al. Rational design and mechanistic insights of advanced photocatalysts for CO2 to C2+ production: Status and challenges[J]. Chinese Journal of Catalysis, 2024, 60(5): 25. (杜晨宇, 盛剑平, 钟丰忆, 等. 光催化二氧化碳还原制多碳产物的先进光催化剂设计与机制: 现状与挑战[J]. 催化学报, 2024, 60(5): 25.DU C Y, SHENG J P, ZHONG F Y, et al. Rational design and mechanistic insights of advanced photocatalysts for CO2 to C2+ production: Status and challenges[J]. Chinese Journal of Catalysis, 2024, 60(5): 25.
|
| [18] |
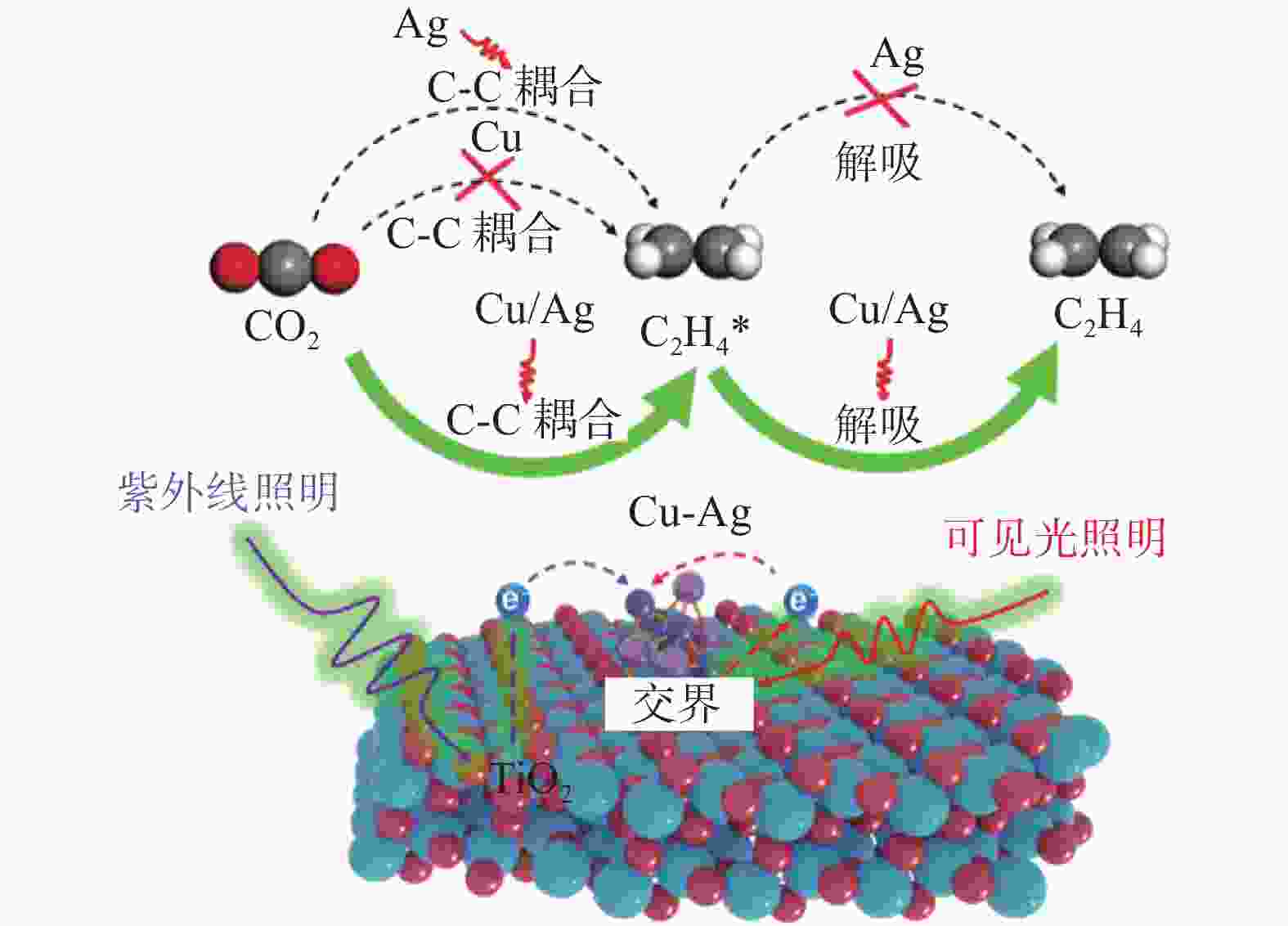
YU Y Y, HE Y, YAN P, et al. Boosted C–C coupling with Cu–Ag alloy sub-nanoclusters for CO2-to-C2H4 photosynthesis[J]. Proceedings of the National Academy of Sciences, 2023, 120(44): e2307320120. doi: 10.1073/pnas.2307320120
|
| [19] |
WU H L, LI X B, TUNG C H, et al. Semiconductor quantum dots: an emerging candidate for CO2 photoreduction[J]. Advanced Materials, 2019, 31(36): 1900709. doi: 10.1002/adma.201900709
|
| [20] |
GUO Q, LIANG F, LI X B, et al. Efficient and selective CO2 reduction integrated with organic synthesis by solar energy[J]. Chem, 2019, 5(10): 2605-2616. doi: 10.1016/j.chempr.2019.06.019
|
| [21] |
BAI Z M, LIU H H, CHEN K Y, et al. Recent progress on chemical conversion of carbon dioxide[J]. Shandong Chemical Industry, 2018, 47(11): 70-72, 76. (白振敏, 刘慧宏, 陈科宇, 等. 二氧化碳化学转化技术研究进展[J]. 山东化工, 2018, 47(11): 70-72, 76. doi: 10.3969/j.issn.1008-021X.2018.11.030BAI Z M, LIU H H, CHEN K Y, et al. Recent progress on chemical conversion of carbon dioxide[J]. Shandong Chemical Industry, 2018, 47(11): 70-72, 76. doi: 10.3969/j.issn.1008-021X.2018.11.030
|
| [22] |
KONG X Y, XIE L, WANG Y M, et al. CO2 capture and resource utilization[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1187-1198. (孔祥宇, 谢亮, 王延民, 等. CO2的捕集及资源化利用[J]. 化工进展, 2022, 41(3): 1187-1198.KONG X Y, XIE L, WANG Y M, et al. CO2 capture and resource utilization[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1187-1198.
|
| [23] |
QIAO J, LIU Y, HONG F, et al. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels[J]. Chemical Society Reviews, 2014, 43(2): 631-675. doi: 10.1039/C3CS60323G
|
| [24] |
ZHENG Y B, ZHANG Q, SHI J, et al. Research progress of catalysts for electrocatalytic reduction of CO2 to various products[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1209-1223. (郑元波, 张前, 石坚, 等. 电催化还原CO2生成多种产物催化剂研究进展[J]. 化工进展, 2022, 41(3): 1209-1223.ZHENG Y B, ZHANG Q, SHI J, et al. Research progress of catalysts for electrocatalytic reduction of CO2 to various products[J]. Chemical Industry and Engineering Progress, 2022, 41(3): 1209-1223.
|
| [25] |
SU W L, FAN Y. Progress of electrocatalytic reduction of CO2 on metal-based materials[J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1384-1394. (苏文礼, 范煜. 金属基材料电催化CO2还原的研究进展[J]. 化工进展, 2021, 40(3): 1384-1394.SU W L, FAN Y. Progress of electrocatalytic reduction of CO2 on metal-based materials[J]. Chemical Industry and Engineering Progress, 2021, 40(3): 1384-1394.
|
| [26] |
CHEN H, LI Z H, ZHANG Z H, et al. Synthesis of composition-tunable syngas from efficiently electrochemical conversion of CO2 over AuCu/CNT bimetallic catalyst[J]. 2019, 58(34): 15425-15431.
|
| [27] |
YAN X, CHEN C, WU Y, et al. Efficient electroreduction of CO2 to C2+ products on CeO2 modified CuO[J]. Chemical science, 2021, 12(19): 6638-6645. doi: 10.1039/D1SC01117K
|
| [28] |
CHEN C J, YAN X P, LIU S J, et al. Highly efficient electroreduction of CO2 to C2+ alcohols on heterogeneous dual active sites[J]. Angewandte Chemie, 2020, 132(38): 16601-16606. doi: 10.1002/ange.202006847
|
| [29] |
GUO L S, SUN J, GE Q J, et al. Recent advances in direct catalytic hydrogenation of carbon dioxide to valuable C2+ hydrocarbons[J]. Journal of Materials Chemistry A, 2018, 6(46): 23244-23262. doi: 10.1039/C8TA05377D
|
| [30] |
JIA J Y, SHAN Y L, TUO Y X, et al. Review of iron-based catalysts for carbon dioxide fischer–tropsch synthesis[J]. transactions of Tianjin University, 2024: 1-20.
|
| [31] |
LI J, HE Y L, TAN L, et al. Integrated tuneable synthesis of liquid fuels via Fischer–Tropsch technology[J]. Nature Catalysis, 2018, 1(10): 787-793. doi: 10.1038/s41929-018-0144-z
|
| [32] |
ZHENG Y H, XU C H, ZHANG X, et al. Synergistic effect of Alkali Na and K promoter on Fe-Co-Cu-Al catalysts for CO2 hydrogenation to light hydrocarbons[J]. Catalysts, 2021, 11(6): 735. doi: 10.3390/catal11060735
|
| [33] |
YANG S, CHUN H J, LEE S, et al. Comparative study of olefin production from CO and CO2 using Na-and K-promoted zinc ferrite[J]. ACS Catalysis, 2020, 10(18): 10742-10759. doi: 10.1021/acscatal.0c02429
|
| [34] |
NASRIDDINOV K, MIN J E, PARK H G, et al. Effect of Co, Cu, and Zn on FeAlK catalysts in CO2 hydrogenation to C5+ hydrocarbons[J]. Catalysis Science & Technology, 2022, 12(3): 906-915.
|
| [35] |
XU Y, ZHAI P, DENG Y C, et al. Highly selective olefin production from CO2 hydrogenation on iron catalysts: a subtle synergy between manganese and sodium additives[J]. Angewandte Chemie, 2020, 132(48): 21920-21928. doi: 10.1002/ange.202009620
|
| [36] |
YANG H Y, DANG Y R, CUI X, et al. Selective synthesis of olefins via CO2 hydrogenation over transition-metal-doped iron-based catalysts[J]. Applied Catalysis B: Environmental, 2023, 321: 122050. doi: 10.1016/j.apcatb.2022.122050
|
| [37] |
TORRES GALVIS H M, DE JONG K P. Catalysts for production of lower olefins from synthesis gas: a review[J]. ACS catalysis, 2013, 3(9): 2130-2149. doi: 10.1021/cs4003436
|
| [38] |
MA G Y, XU Y F, WANG J, et al. Research progress of iron-based catalyst for converting syngas directly to light olefins[J]. Chemical Industry and Engineering Progress, 2018, 37(3): 992-1000. (马光远, 徐艳飞, 王捷, 等. 合成气直接法制取低碳烯烃铁基催化体系研究进展[J]. 化工进展, 2018, 37(3): 992-1000.MA G Y, XU Y F, WANG J, et al. Research progress of iron-based catalyst for converting syngas directly to light olefins[J]. Chemical Industry and Engineering Progress, 2018, 37(3): 992-1000.
|
| [39] |
HE X, CHEN D R, WANG W N. Bimetallic metal-organic frameworks (MOFs) synthesized using the spray method for tunable CO2 adsorption[J]. Chemical Engineering Journal, 2020, 382: 122825. doi: 10.1016/j.cej.2019.122825
|
| [40] |
ZENG G T, QIU J, LI Z, et al. CO2 reduction to methanol on TiO2-passivated GaP photocatalysts[J]. ACS Catalysis, 2014, 4(10): 3512-3516. doi: 10.1021/cs500697w
|
| [41] |
CIMINO S, BOCCIA F, LISI L. Effect of alkali promoters (Li, Na, K) on the performance of Ru/Al2O3 catalysts for CO2 capture and hydrogenation to methane[J]. Journal of CO2 Utilization, 2020, 37: 195-203. doi: 10.1016/j.jcou.2019.12.010
|
| [42] |
WANG Z Q, XU Z N, PENG S Y, et al. High-performance and long-lived Cu/SiO2 nanocatalyst for CO2 hydrogenation[J]. ACS Catalysis, 2015, 5(7): 4255-4259. doi: 10.1021/acscatal.5b00682
|
| [43] |
WANG S W, WU T J, LIN J, et al. Iron–potassium on single-walled carbon nanotubes as efficient catalyst for CO2 hydrogenation to heavy olefins[J]. Acs Catalysis, 2020, 10(11): 6389-6401. doi: 10.1021/acscatal.0c00810
|
| [44] |
JOHNSON G R, BELL A T. Role of ZrO2 in promoting the activity and selectivity of Co-based fischer–tropsch synthesis catalysts[J]. ACS Catalysis, 2016, 6(1): 100-114. doi: 10.1021/acscatal.5b02205
|
| [45] |
WU T J, LIN J, CHENG Y, et al. Porous graphene-confined Fe–K as highly efficient catalyst for CO2 direct hydrogenation to light olefins[J]. ACS applied materials & interfaces, 2018, 10(28): 23439-23443.
|
| [46] |
XU M J, ZHU M H, CHEN T Y, et al. High value utilization of CO2: research progress of catalyst for hydrogenation of CO2 to methanol[J]. Chemical Industry and Engineering Progress, 2021, 40(2): 565-576. (徐敏杰, 朱明辉, 陈天元, 等. CO2高值化利用: CO2加氢制甲醇催化剂研究进展[J]. 化工进展, 2021, 40(2): 565-576.XU M J, ZHU M H, CHEN T Y, et al. High value utilization of CO2: research progress of catalyst for hydrogenation of CO2 to methanol[J]. Chemical Industry and Engineering Progress, 2021, 40(2): 565-576.
|
| [47] |
SUN K H, ZHANG Z T, SHEN C Y, et al. The feasibility study of the indium oxide supported silver catalyst for selective hydrogenation of CO2 to methanol[J]. Green Energy & Environment, 2022, 7(4): 807-817.
|
| [48] |
WU C Y, LIN L L, LIU J J, et al. Inverse ZrO2/Cu as a highly efficient methanol synthesis catalyst from CO2 hydrogenation[J]. Nature communications, 2020, 11(1): 5767. doi: 10.1038/s41467-020-19634-8
|
| [49] |
LI M M J, ZENG Z Y, LIAO F L, et al. Enhanced CO2 hydrogenation to methanol over CuZn nanoalloy in Ga modified Cu/ZnO catalysts[J]. Journal of Catalysis, 2016, 343: 157-167. doi: 10.1016/j.jcat.2016.03.020
|
| [50] |
SUN X, LI H. Recent progress of Ga-based liquid metals in catalysis[J]. RSC advances, 2022, 12(38): 24946-24957. doi: 10.1039/D2RA04795K
|




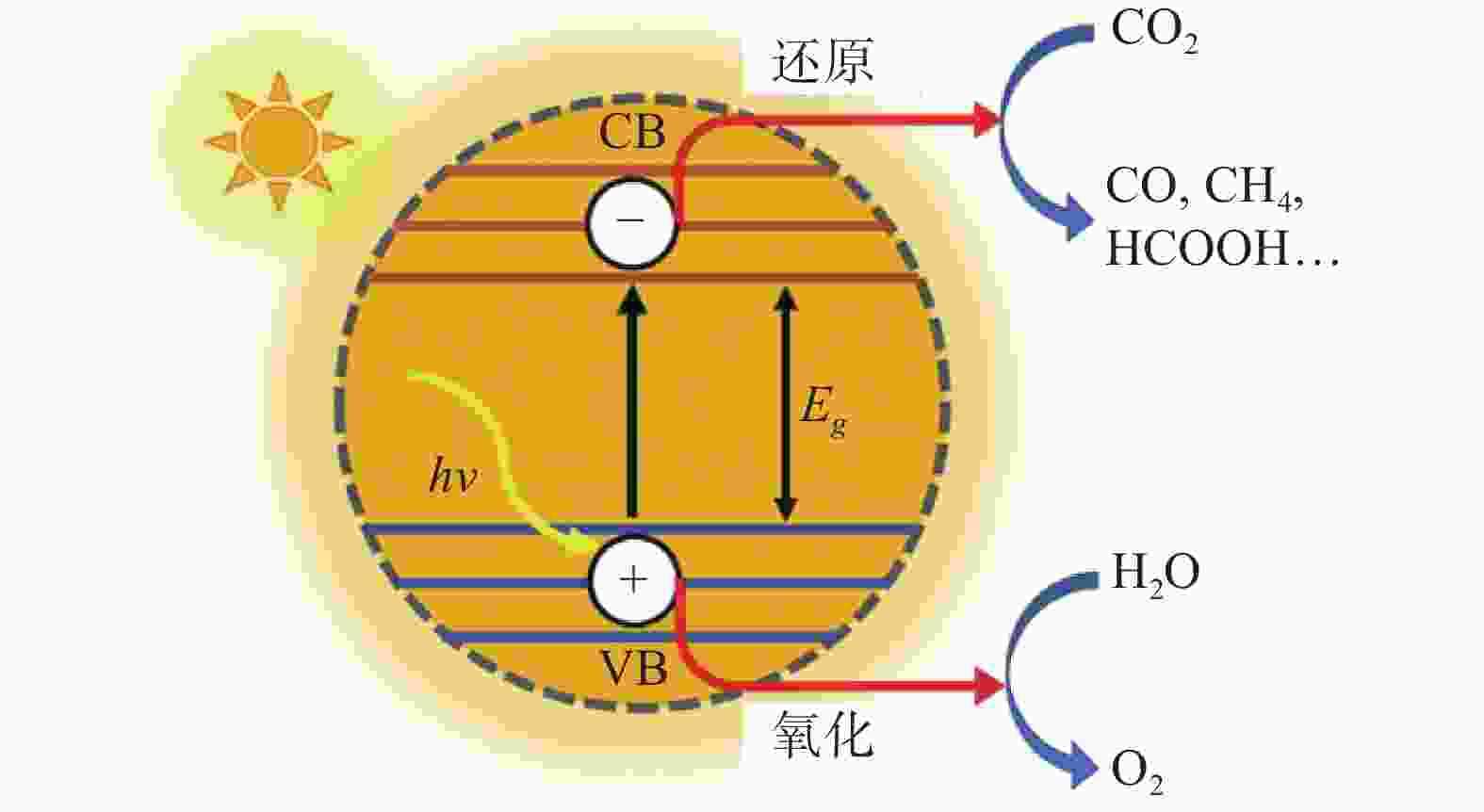
 下载:
下载: