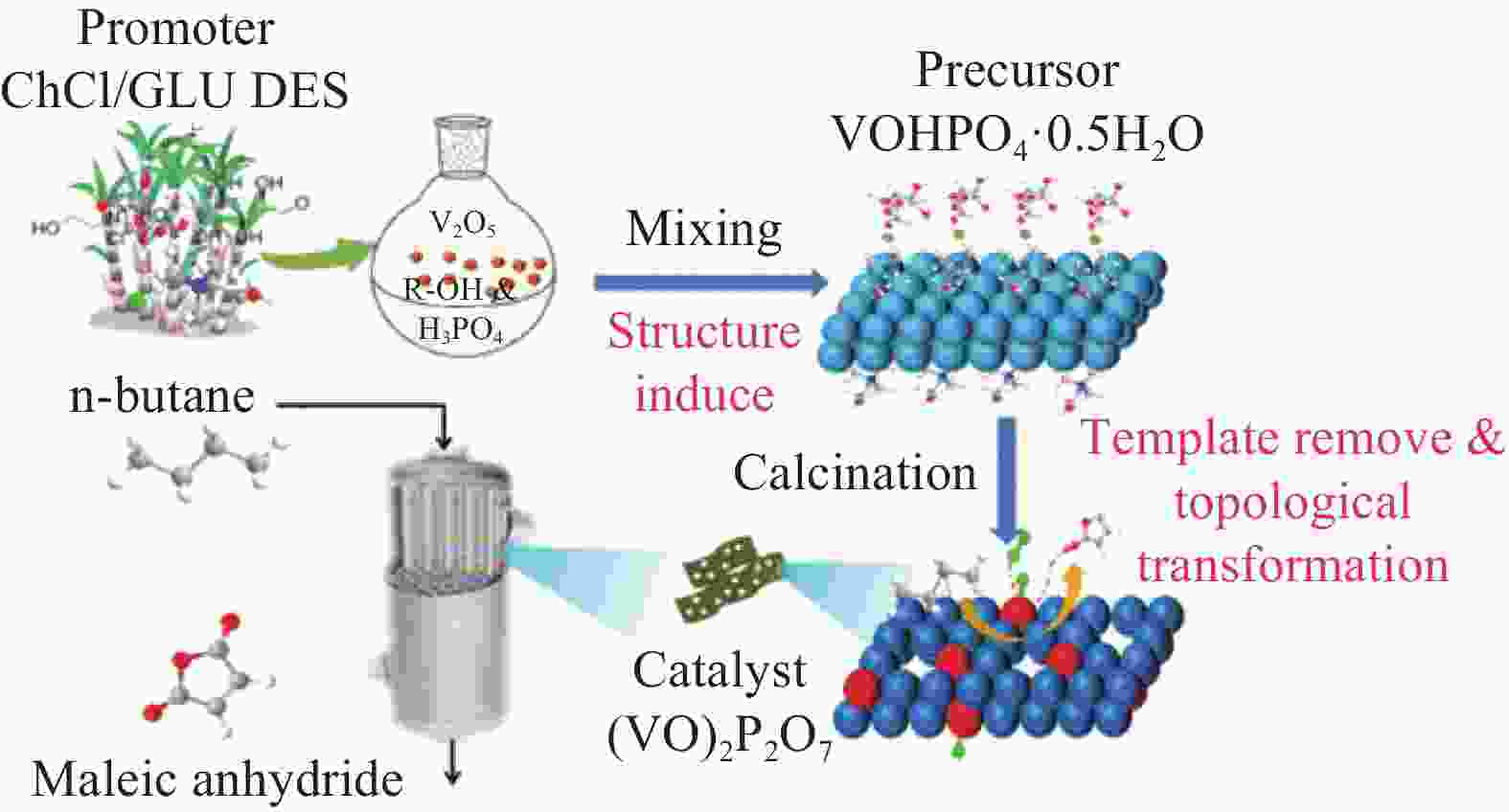
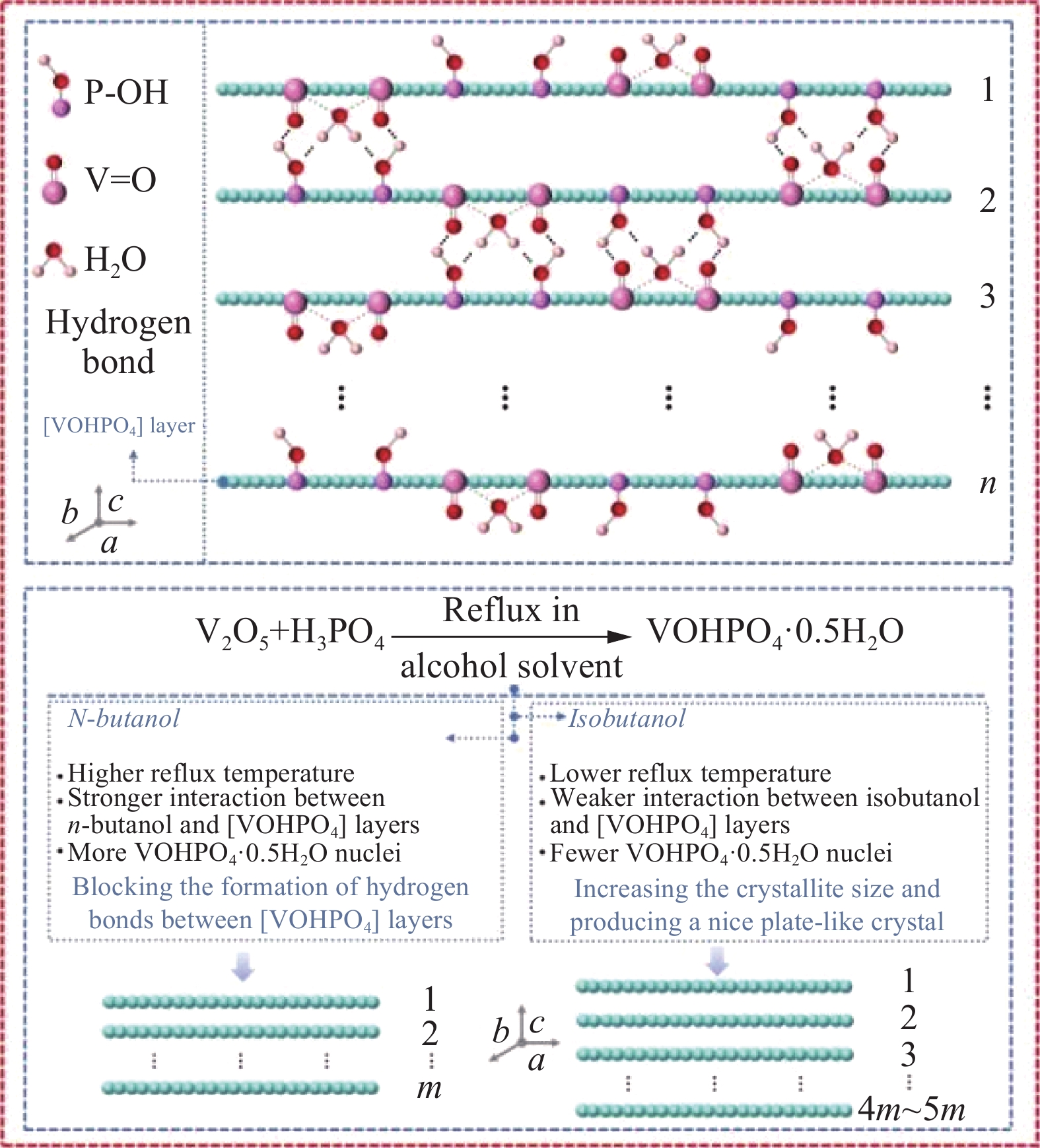
Progress and prospects of vanadium application in vanadium-phosphorus-oxygen catalysts for maleic anhydride
-
摘要: 钒磷氧(VPO)催化剂作为正丁烷法制备顺酐的核心催化剂,其较低的正丁烷转化率和顺酐选择性难以满足工业对顺酐高效生产的需求,因此开发高性能钒磷氧催化剂成为研究热点。基于此,综述了近年来高效钒磷氧催化剂的制备进展,重点探讨了原料与溶剂选择、制备方法、活化气氛、助剂及载体对催化性能的影响,发现上述因素主要通过改变催化剂比表面积、活性晶面强度、表面酸性、V4+/V5+或P/V比,使其暴露更多活性位点,促进正丁烷C-H键断裂和诱导正丁烷发生氧化而提高正丁烷转化率或顺酐选择性,最后,总结对比了不同影响因素对VPO催化性能的影响,提出添加助剂是制备高性能VPO催化剂的发展趋势,并从原料选择、结构设计与改性和成本等角度对未来助剂发展进行展望。
-
关键词:
- 正丁烷氧化 /
- 顺酐 /
- 钒磷氧催化剂 /
- 催化性能 /
- 前体VOHPO4·0.5H2O
Abstract: As the core catalyst for maleic anhydride in the n-butane process, vanadium-phosphorus-oxygen (VPO) catalysts, with their low n-butane conversion and maleic anhydride selectivity, can hardly satisfy the industrial demand for the efficient production of maleic anhydride, and therefore the development of high-performance vanadium-phosphorus-oxygen (VPO) catalysts has become a hot spot in research. On this backgroud, we have reviewed in this paper the progress of the preparation of high-efficiency vanadium-phosphorus-oxygen catalysts in recent years, and have discussed particularly on the effects of raw material and solvent selection, preparation method, activation atmosphere, additives and carriers on the catalytic performance. We have found that the factors mentioned above modify mainly the catalysts by altering the catalyst's specific surface area, active crystal surface strength, surface acidity, V4+/V5+ or P/V ratio. These modifications help to expose more active sites, to promote n-butane C-H bond breaking and to induce the oxidation of n-butane to improve the n-butane conversion or maleic anhydride selectivity. Finally, we have summarized and compared the effects of different influencing factors on the catalytic performance of VPO, and have suggested that the addition of additives is the development trend for the preparation of high-performance VPO catalysts. We look forward to the development of additives in the future in terms of the selection of raw materials, the structural design and modification, and the cost. -
表 1 原料选择和制备过程对正丁烷氧化制顺酐性能影响
Table 1. Influence of raw material selection and preparation process on the transformation of n-butane oxidation to maleic anhydride
Catalyst Reaction
temperature/℃Reaction atmosphere/vol % GHSV/h−1 Conversion of
n-butane/%MA selecti-
vity/%Ref. n-Butane Air Oxygen Nitrogen Argon Vapor VPOOr 1.0 370 2.8 97.2 4200 63 97 [6] VPO(P/V=0.93) 381 1.2 98.8 400 40 70 [8] VPO-T 420 1.36 18.92 79.72 2000 91.4 62.7 [9] VPO-0.67 mol/L 420 1.36 18.92 79.72 2000 >81 >66 [9] VPO-200 W 420 1.36 18.92 79.72 2000 87.2 70.2 [9] Evaluated Catalyst 400 1.392 98.608 2000 88.2 64.33 [10] VPOS30KCl 400 1.0 99.0 2400 19 48 [11] VPO-Vsg 400 1.57 21.0 13.7 63.73 2000 90 70 [12] VPO2 400 1.57 21.0 13.7 63.73 2000 65 54 [14] VPOA1 400 1.7 98.3 2400 53 65 [14] VPO-80% 400 1.7 98.3 2000 87 64 [15] VPOscc 400 1.5 98.5 2400 24 48 [18] VPO-1A 412 1.4271 98.6729 1560 >85 >65 [22] V-P-O 410 1.0 84.0 15.0 17.5 57 [24] SZ-27% 400 1.4 98.6 1200 89.47 71.97 [25] 表 2 不同助剂对正丁烷氧化制顺酐影响
Table 2. Effects of different additives on the oxidation of n-butane to maleic anhydride
Catalyst Metal element Reaction
temperature/℃Reaction atmosphere/vol % GHSV/h−1 Conversion of
n-butane/%MA
selectivity/%Ref. n-Butane Air Oxygen Nitrogen 1Sb2O3-O@VPO Sb 420 1.45 98.55 2000 80.84 70.18 [20] VPOs-Bi5% Bi 400 1.0 99.0 2400 29 86 [27] NanoVPP1%Ce Ce 400 1.0 99.0 2400 65 51 [28] VPOD1
(VPO/Ce+Bi)Ce+Bi 400 1.7 98.3 2400 78 67 [29] V-P-O-Mn
(atomic% Mn/Mn+V=0.1)Mn 400 1.5 19.7 78.8 3000 51.8 60.5 [30] V-P-O-Zn
(atomic% Zn/Zn+V=0.05)Zn 400 1.5 19.7 78.8 3000 61.4 64.4 [30] VNbPO Nb 400 1.6 98.4 2000 75 70.1 [31] VPDTe Te 400 1.7 98.3 2400 80 32 [32] 3%Sm-VPO Sm 420 1.5 98.5 3000 87 67 [33] Y-VPO Y 430 1.1 98.9 2000 92.37 60.93 [34] Sc-VPO Sc 430 1.1 98.9 2000 96.44 47.89 [34] 0.01Y-VPO Y 430 1.5 98.5 2000 86.9 71.5 [35] VPO-Cu Cu 380 1.5 19.3 79.2 3000 90.3 63.2 [36] VPCo4-Iaa Co 430 1.5 98.5 2000 80 60 [37] PMA-VPP Mo 420 1.35 98.65 2000 99.1 60 [38] Cr1.0i Cr 380 1.5 98.5 900 80 70 [39] VPO-BMIMFeCl4 Fe 420 1.40 98.6 2000 91.60 65.88 [40] VPO-OMIMFeCl4 Fe 420 1.40 98.6 2000 87.77 66.67 [40] [TBA]PMoV@VPO Mo 420 1.34 98.66 2000 95.20 58.30 [41] 3%PIL-VPO 420 1.50 98.5 2000 88.10 67.20 [42] Zr-DES-VPO Zr 430 1.34 98.66 2000 96.53 53.48 [43] Zr-Mo-DES-VPO Zr+Mo 430 1.34 98.66 2000 74.77 49.94 [43] VPO-MgCl2/EG Mg 430 1.36 18.2 80.44 2000 86.19 >62 [44] VPO-DES-0.4 420 1.50 98.5 2000 92.23 60.80 [45] VPO-DES-0.6 420 1.50 98.5 2000 92.48 60.13 [46] VPO-CeNN (1:0.5) Ce 420 1.34 98.66 2000 90.95 59.25 [47] 表 3 不同影响因素对VPO性能影响及其优劣势情况
Table 3. Impact of different influencing factors on VPO performance, as well as advantages and disadvantages analysis
Factor Conditions Effect on physicochemical properties Advantages Disadvantages Vanadium source VCl3, VOSO4, VO(acac)2, V2O5 with modified material V4+/V5+ ratio,
active plane intensity,
lattice oxygen/surface oxygen ratioBroad availability, cost-effective Potential impurity contamination Synthesis method Liquid-phase, microwave, solvothermal method Raw material dispersion, crystallinity,
specific surface areaVarious options,
high scalabilityLow reproducibility Alcohol solvent Alcohols (different chain length or isomer variation) Morphological,
crystallinityEnvironmentally benign Residual solvent,
volatile solvents,
variable costActivation conditions Atmosphere (air/N2 ratio, H2O presence) P/V ratio, surface acidity,
vanadium oxidation stateAdjustable,
flexibleHigh energy input and equipment requirements Metal additives Rare earth metals,
transition metals,
alkaline earth metalsSpecific surface area,
V4+/V5+ ratio,
crystallinityAdjustable,
various options,
good modificationPotential impurity contamination Ionic liquid or DES Organic cations + metal anions;
H-bond donors+acceptorsMorphology,
specific surface area,
active plane intensity,Functional design, eco-friendly High synthesis cost,
intricate reaction pathwaysSupport material Metal oxides,
2D compounds material,
carbon materialSpecific surface area Enhanced structural integrity Uneven catalyst dispersion,
increased synthesis complexity -
[1] MULLER M, KUTSCHERAUER M, BOCKLEIN S, et al. Modeling the selective oxidation of n-butane to maleic anhydride: from active site to industrial reactor. Catalysis Today, 2022, 387, 82-106. [2] SCHULZ C, ROY S C, WITTICH K, et al. αII-(V1-xWx)OPO4 catalysts for the selective oxidation of n-butane to maleic anhydride. Catalysis Today, 2019, 333, 113-119. [3] JIA X F, ZHANG D S. Progresses in research for VPO catalysts used in selective oxidation of n-butane to maleic anhydride[J]. Petrochemical Technology, 2016, 45(6): 749-755. (贾雪飞, 张东顺. 正丁烷选择氧化制顺酐钒磷氧催化剂晶相结构的研究进展[J]. 石油化工, 2016, 45(6): 749-755.JIA X F, ZHANG D S. Progresses in research for VPO catalysts used in selective oxidation of n-butane to maleic anhydride[J]. Petrochemical Technology, 2016, 45(6): 749-755. [4] ZHANG Q, FANG W G, LI G X. Research progress on new preparation methods of vanadium phosphorus oxide catalysts[J]. Rare Metals and Cemented Carbides, 2017, 45(1): 63-66. (张琪, 方伟国, 李贵贤. 钒磷氧催化剂的新制备方法研究进展[J]. 稀有金属与硬质合金, 2017, 45(1): 63-66.ZHANG Q, FANG W G, LI G X. Research progress on new preparation methods of vanadium phosphorus oxide catalysts[J]. Rare Metals and Cemented Carbides, 2017, 45(1): 63-66. [5] LIU R X, HE B, LUO C, et al. Vanadium phosphorous oxide and its catalytic application[J]. CIESC Journal, 2018, 69(4): 1261-1275. (刘瑞霞, 贺滨, 罗琛, 等. 钒磷氧复合氧化物及其在催化领域的应用[J]. 化工学报, 2018, 69(4): 1261-1275.LIU R X, HE B, LUO C, et al. Vanadium phosphorous oxide and its catalytic application[J]. CIESC Journal, 2018, 69(4): 1261-1275. [6] BATIS N H, BATIS H, GHORBEL A, et al. Synthesis and characterization of new VPO catalysts for partial n-butane oxidation to maleic anhydride[J]. Journal of Catalysis, 1991, 128 (1), 248-263. [7] MIZUNO N, HATAYAMA H, MISONO M. One-pot synthesis of VOHPO4·0.5H2O with high growth of the (001) plane: an important catalyst precursor of (VO)2P2O7[J]. Chemistry of Materials, 1997, 9 (12), 2697-2698. [8] GULIANTS V V, BENZIGER J B, SUNDARESAN S. New layered vanadyl(IV) phosphite as a precursor to vanadyl pyrophosphate catalysts for partial oxidation of n-butane to maleic anhydride[J]. Journal of Catalysis, 1995, 156 (2), 298-300. [9] XIE Z Q, ZHU S W, WANG X S, et al. Microwave synthesis of vanadium phosphorus oxide catalysts for n-butane selective oxidation[J]. Particuology, 2025, 96, 97-105. [10] ZHANG X, WANG H B, GOU L K, et al. Reaction behaviors and crystal transformation of industrial vanadium-phosphorus-oxygen (VPO) catalysts for n-butane oxidation[J]. ACS Omega, 2021, 6 (36), 23558-23563. [11] TAUFIQ-YAP Y H, HOH J R J, WONG Y C. Synthesis of nanostructured vanadium phosphate catalysts using sonochemical route for partial oxidation of n-butane[J]. Journal of Applied Sciences, 2011, 11 (13), 2370-2375. [12] ZHANG X M, ZHAO Z K. An efficient vanadium phosphorus oxide catalyst prepared by tuning vanadium precursor for selective oxidation of n-butane to maleic anhydride[J]. Materials Letters, 2024, 357, 135679. [13] O'MAHONY L. Crystallisation of VOHPO4·0.5H2O[J]. Applied Catalysis A: General, 2003, 253 (2), 409-416. [14] ROWNAGHI A A, TAUFIQ-YAP Y H. Novel synthesis techniques for preparation of ultrahigh-crystalline vanadyl pyrophosphate as a highly selective catalyst for n-butane oxidation[J]. Industrial & Engineering Chemistry Research, 2010, 49 (5), 2135-2143. [15] XU J L, LI N, LI X Y, et al. Role of regulating synthetic solvents in enhancing the catalytic performance of VPO catalysts for n-butane oxidation to maleic anhydride[J]. Chemical Engineering Journal, 2024, 496, 153635. [16] AIT-LACHGAR K, TUEL A, BRUN M, et al. Selective oxidation of n-butane to maleic anhydride on vanadyl pyrophosphate: II. Characterization of the oxygen-treated catalyst by electrical conductivity, Raman, XPS, and NMR spectroscopic techniques[J]. Journal of Catalysis, 1998, 177 (2), 224-230. [17] FAIZAN M, ZHANG R R, LIU R X. Vanadium phosphorus oxide catalyst: Progress, development and applications[J]. Journal of Industrial and Engineering Chemistry, 2022, 110, 27-67. [18] HUTCHINGS G J, BARTLEY J K, WEBSTER J M, et al. Amorphous vanadium phosphate catalysts from supercritical antisolvent precipitation[J]. Journal of Catalysis, 2001, 197 (2), 232-235. [19] FAIZAN M, AAMIE E, KIONG T S, et al. Role of the catalyst structure-activity relationship in enhancing the selective oxidation yield of n-butane to maleic anhydride[J]. Catalysis Science & Technology, 2024, 14 (17), 5009-5031. [20] ZHAO J Y, ZHANG J, YANG F W, et al. Regulation of maleic anhydride selectivity for n-butane oxidation by Sb2O3-modified vanadium phosphorus oxide catalysts[J]. Industrial & Engineering Chemistry Research, 2024, 63 (26), 11392-11403. [21] XU L, TONG M Y, WANG H B. Influence of activation conditions on physical properties and reaction performance of vanadium-phosphorous oxide catalyst[J]. Contemporary Chemical Industry, 2011, 40(11): 1128-1130, 1142. (许磊, 佟明友, 王海波. 活化条件对钒磷氧催化剂物性及反应性能的影响[J]. 当代化工, 2011, 40(11): 1128-1130, 1142.XU L, TONG M Y, WANG H B. Influence of activation conditions on physical properties and reaction performance of vanadium-phosphorous oxide catalyst[J]. Contemporary Chemical Industry, 2011, 40(11): 1128-1130, 1142. [22] ZHANG X, WANG H B, GOU L K, et al. Synthesis of an efficient VPO catalyst for the selective oxidation of n-butane to the MA product: mechanism of crystal transformation[J]. Chemical Engineering Science, 2023, 274, 118708. [23] RICHTER F, PAPP H, GOTZE T, et al. Investigation of the surface of vanadyl pyrophosphate catalysts[J]. Surface and Interface Analysis, 1998, 26 (10), 736-741. [24] ARNOLD E W, SUNDARESAN S. Effect of water vapor on the activity and selectivity characteristics of a vanadium phosphate catalyst towards butane oxidation[J]. Applied Catalysis, 1988, 41, 225-239. [25] ZHANG Y K, ZHANG R R, DONG J, et al. The critical role of steam during activation process on the catalytic performance of VPO for n-butane selective oxidation to maleic anhydride[J]. Journal of Catalysis, 2022, 416, 157-169. [26] HUTCHINGS G J. Effect of promoters and reactant concentration on the selective oxidation of n-butane to maleic anhydride using vanadium phosphorus oxide catalysts[J]. Applied Catalysis, 1991, 72 (1), 1-32. [27] LEONG L K, CHIN K S, TAUFIQ-YAP Y H. The effect of Bi promoter on vanadium phosphate catalysts synthesized via sesquihydrate route[J]. Catalysis Today, 2011, 164 (1), 341-346. [28] TAUFIQ-YAP Y H, NURUL S N M, HUSSEIN M Z. Influences of the various metal dopants for the nanosized vanadium phosphate catalysts[J]. Catalysis Letters, 2011, 141 (1), 136-148. [29] ROWNAGHI A A, TAUFIQ-YAP Y H, REZAEI F. Influence of rare-earth and bimetallic promoters on various VPO catalysts for partial oxidation of n-butane[J]. Catalysis Letters, 2009, 130 (3), 504-516. [30] TAKITA Y, TANAKA K, ICHIMARU S, et al. Incorporation of promoter elements into the crystal lattice of (VO)2P2O7 and its promotion effects on the oxidation of n-butane to maleic anhydride[J]. Applied Catalysis A: General, 1993, 103 (2), 281-290. [31] DUARTE D F A M, GONZALEZ W D A, PRIES D O P G, et al. Vanadium phosphorus oxide catalyst modified by niobium doping for mild oxidation of n-butane to maleic anhydride[J]. Journal of Catalysis, 2002, 208 (1), 238-246. [32] TAUFIQ-YAP Y H, ASRINA S N, HUTCHINGS G J, et al. Effect of tellurium promoter on vanadium phosphate catalyst for partial oxidation of n-butane[J]. Journal of Natural Gas Chemistry, 2011, 20 (6), 635-638. [33] WU H Y, WANG H B, LIU X H, et al. Samarium-modified vanadium phosphate catalyst for the selective oxidation of n-butane to maleic anhydride[J]. Applied Surface Science, 2015, 351, 243-249. [34] LI W J, GUO S P, GUO H Q, et al. Effect of Ⅲ B metals (Sc, Y, La and Ce) on active components regulation of VPO catalyst in n-butane selective oxidation[J]. Molecular Catalysis, 2023, 547, 113318. [35] LI W J, XIAO Y, GUO S P, et al. Increasing maleic anhydride selectivity for n-butane oxidation by Y-modified VPO catalysts[J]. Fuel, 2023, 333, 126214. [36] YE D Q, FU M L, TIAN L Q, et al. Effect of promoters on the behavior and properties of VPO catalysts for the selective oxidation of n-butane to maleic anhydride[J]. Research on Chemical Intermediates, 2003, 29 (3), 271-284. [37] CORNAGLIA L, IRUSTA S, LOMBARDO E A, et al. The beneficial effect of cobalt on VPO catalysts[J]. Catalysis Today, 2003, 78 (1), 291-301. [38] HE B, NAN L L, LI Z H, et al. Effect of Mo species on the selective oxidation of n-butane to maleic anhydride over Mo-promoted VPP[J]. ChemistrySelect, 2019, 4 (2), 662-669. [39] PIERINI B T, LOMBARDO E A. Structure and properties of Cr promoted VPO catalysts[J]. Materials Chemistry and Physics, 2005, 92 (1), 197-204. [40] DAI F, LI Z H, CHEN X J, et al. Synthesis of vanadium phosphorus oxide catalysts promoted by iron-based ionic liquids and their catalytic performance in selective oxidation of n-butane[J]. Catalysis Science & Technology, 2018, 8 (17), 4515-4525. [41] LI K, HE B, LIU J C, et al. Synergistic interaction of anions and cations in preparation of VPO catalysts promoted by polyoxometalate-ionic liquids[J]. Applied Catalysis A: General, 2019, 582, 117106. [42] DAI F, SHI Y N, ZHANG T, et al. Phosphorus-based ionic liquid as dual function promoter oriented synthesis of efficient VPO catalyst for selective oxidation of n-butane. Catalysis Letters, 2021, 151 (1), 255-266. [43] FAIZAN M, NIAZI K U K, NAWAZ H, et al. Mono-, bi-, and tri-metallic DES are prepared from Nb, Zr, and Mo for n-butane selective oxidation via VPO catalyst. Processes, 2021, 9(9), 1487. [44] WANG X S, CHANG Z, WU L Z, et al. Preparation of enhanced vanadium phosphorus oxide catalysts by alkaline earth metal-alcohols deep eutectic solvents[J]. The Chinese Journal of Process Engineering, 2023, 23(1): 98-106. (王兴盛, 常智, 吴刘柱, 等. 碱土金属醇类低共熔溶剂强化钒磷氧催化剂的制备及催化性能研究[J]. 过程工程学报, 2023, 23(1): 98-106.WANG X S, CHANG Z, WU L Z, et al. Preparation of enhanced vanadium phosphorus oxide catalysts by alkaline earth metal-alcohols deep eutectic solvents[J]. The Chinese Journal of Process Engineering, 2023, 23(1): 98-106. [45] HE B, LI Z H, ZHANG H L, et al. Synthesis of vanadium phosphorus oxide catalysts assisted by deep-eutectic solvents for n-butane selective oxidation[J]. Industrial & Engineering Chemistry Research, 2019, 58 (8), 2857-2867. [46] HE B, LI Y W, ZHANG T, et al. Synthesis of porous and highly crystallinity vanadium phosphorus oxide catalysts by multifunctional biomass-based deep eutectic solvents[J]. The Journal of Physical Chemistry B, 2020, 124 (18), 3743-3753. [47] FAIZAN M, LI Y W, WANG X S, et al. Rare earth metal based DES assisted the VPO synthesis for n-butane selective oxidation toward maleic anhydride[J]. Green Energy & Environment, 2023, 8 (6), 1737-1752. [48] NIE W Y, WANG Z Y, JI W J, et al. Comparative studies on the VPO specimen supported on mesoporous Al-containing MCM-41 and large-pore silica. Applied Catalysis A: General, 2003, 244 (2), 265-272. [49] LI X K, JI W J, ZHAO J, et al. n-Butane oxidation over VPO catalysts supported on SBA-15. Journal of Catalysis, 2006, 238 (1), 232-241. [50] GUERRERO-PEREZ M O, BERENGUER R, FORD M E, et al. Carbon-supported VPO catalysts with fibrous structure: A new family of catalysts for partial oxidation reactions. Catalysis Today, 2023, 423, 114291. [51] WU H Y, JIN P, SUN Y F, et al. Enhancing catalytic performance of phosphorus-modified ceria supported VPO catalysts for n-butane oxidation[J]. Journal of Molecular Catalysis A: Chemical, 2016, 414, 1-8. -




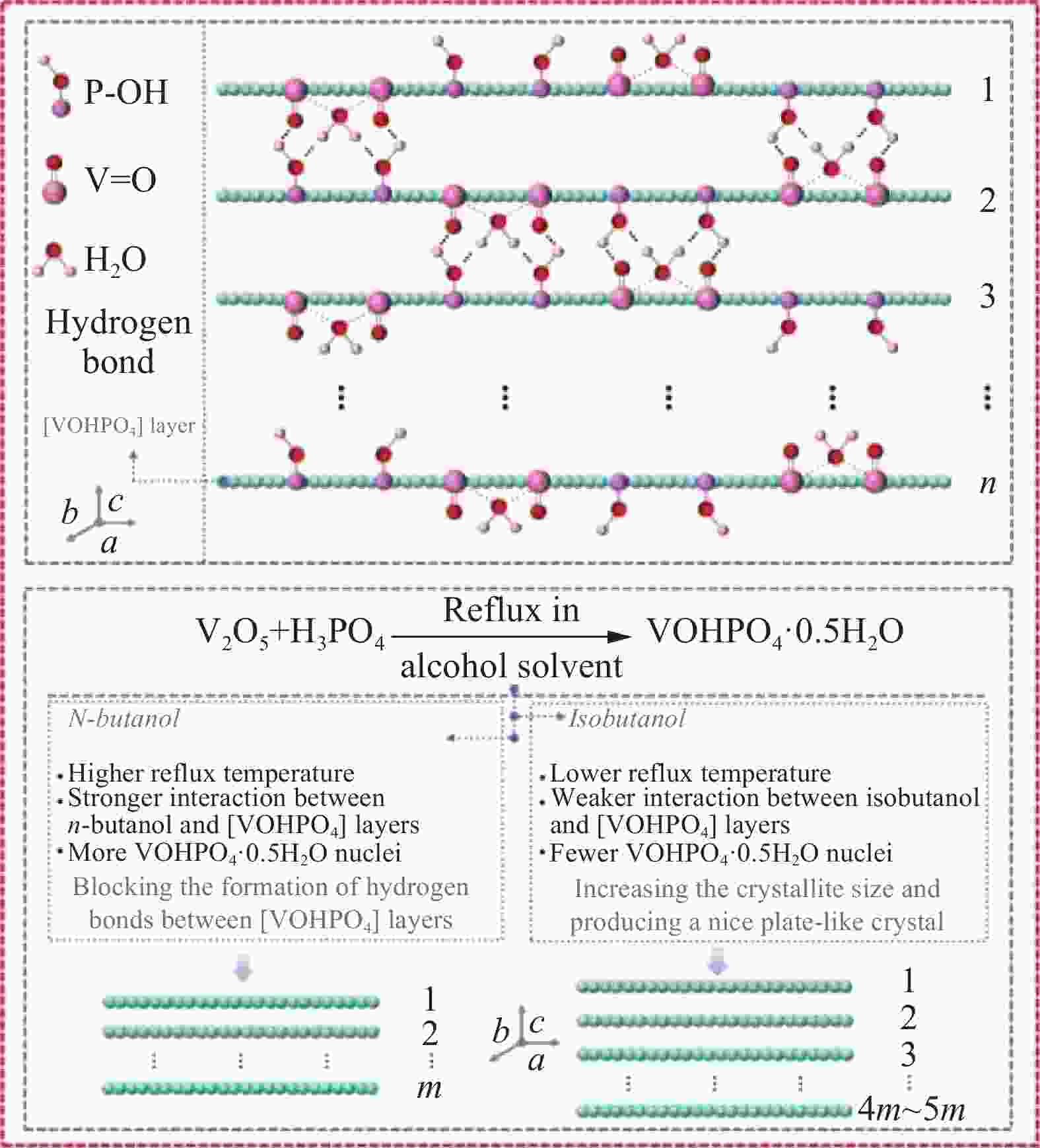
 下载:
下载: