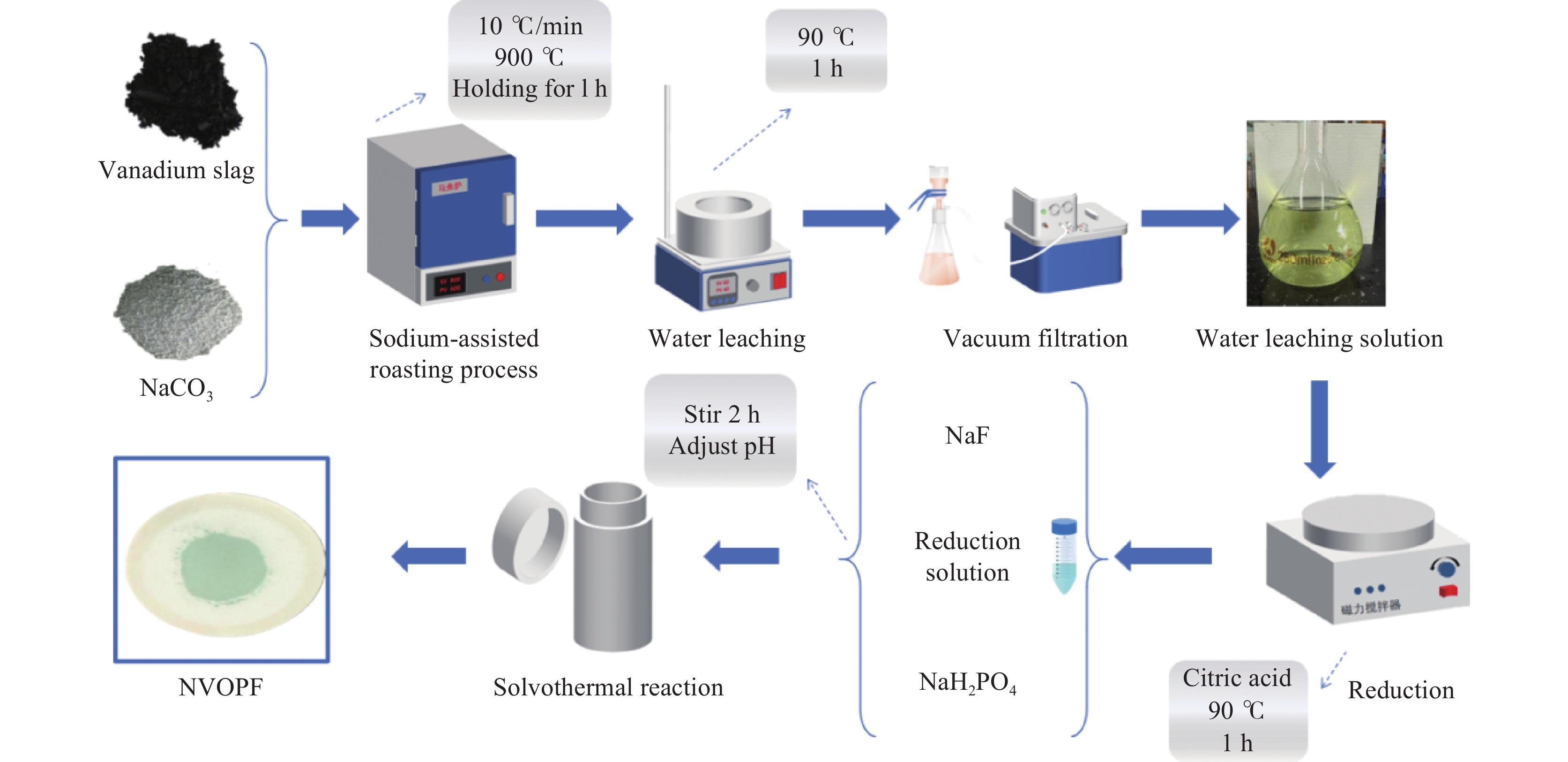
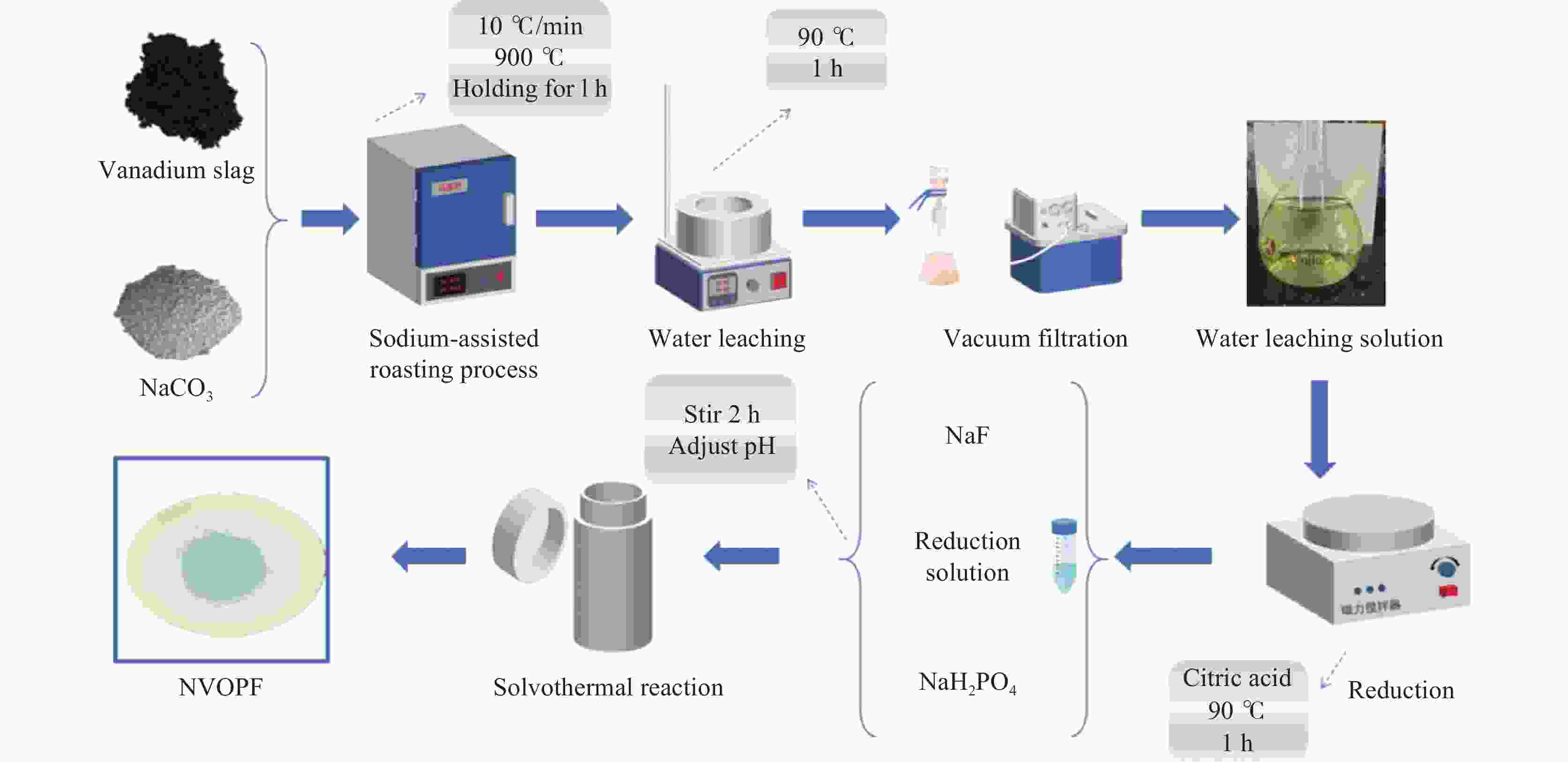
In-situ preparation of sodium vanadium fluorophosphate from sodiumized vanadium slag leaching solution and its performance study
-
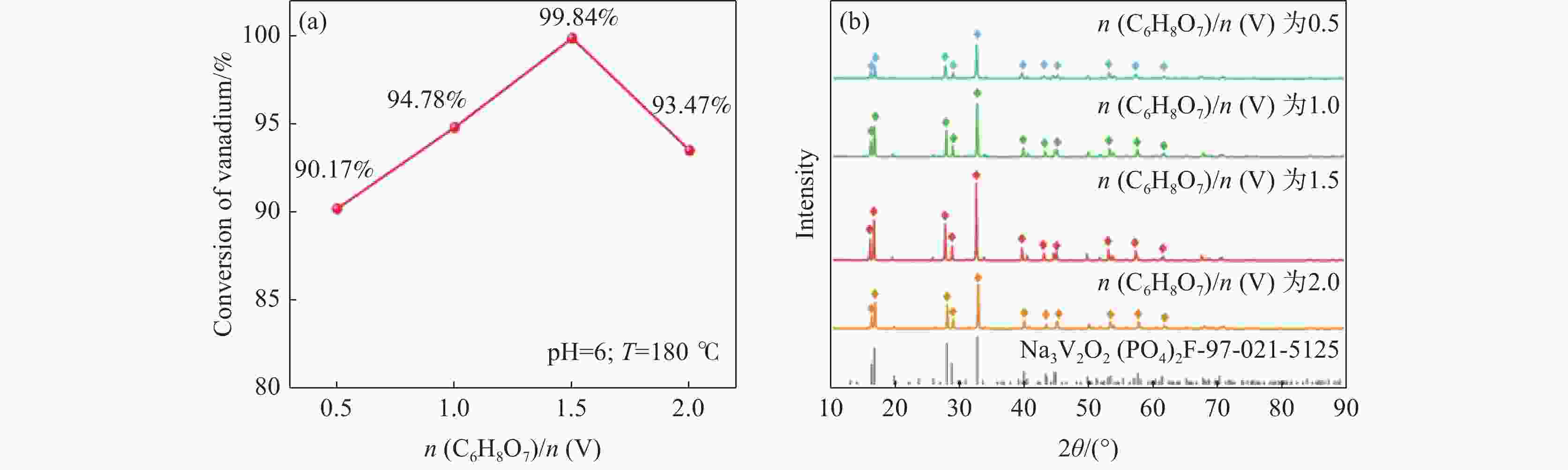
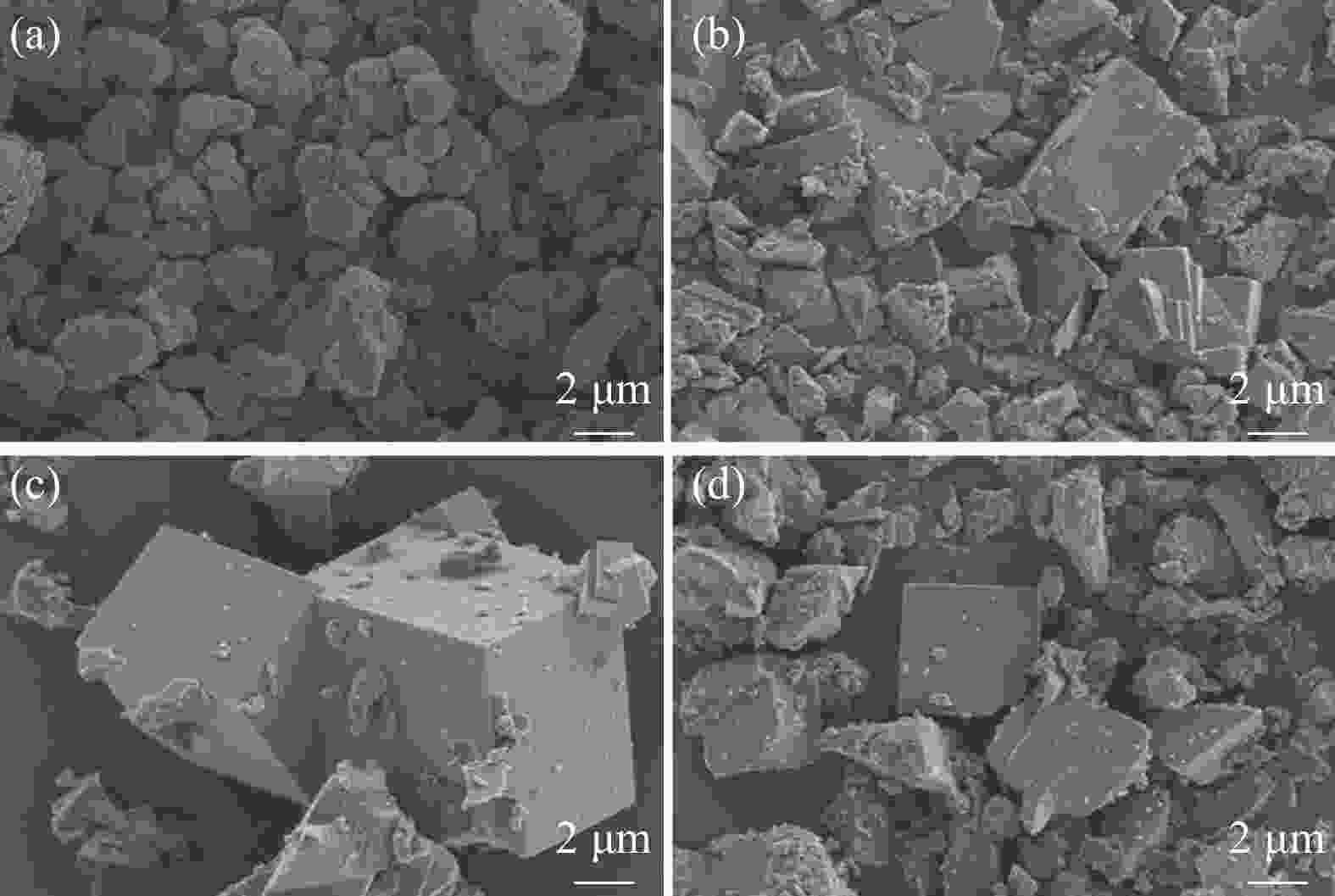
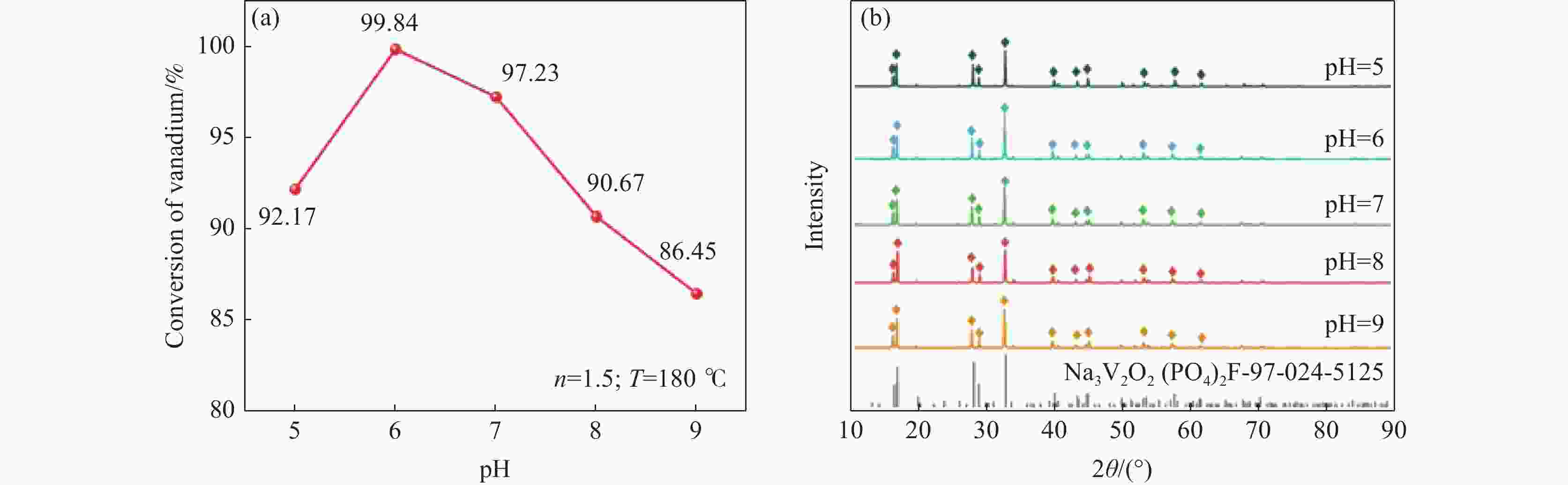
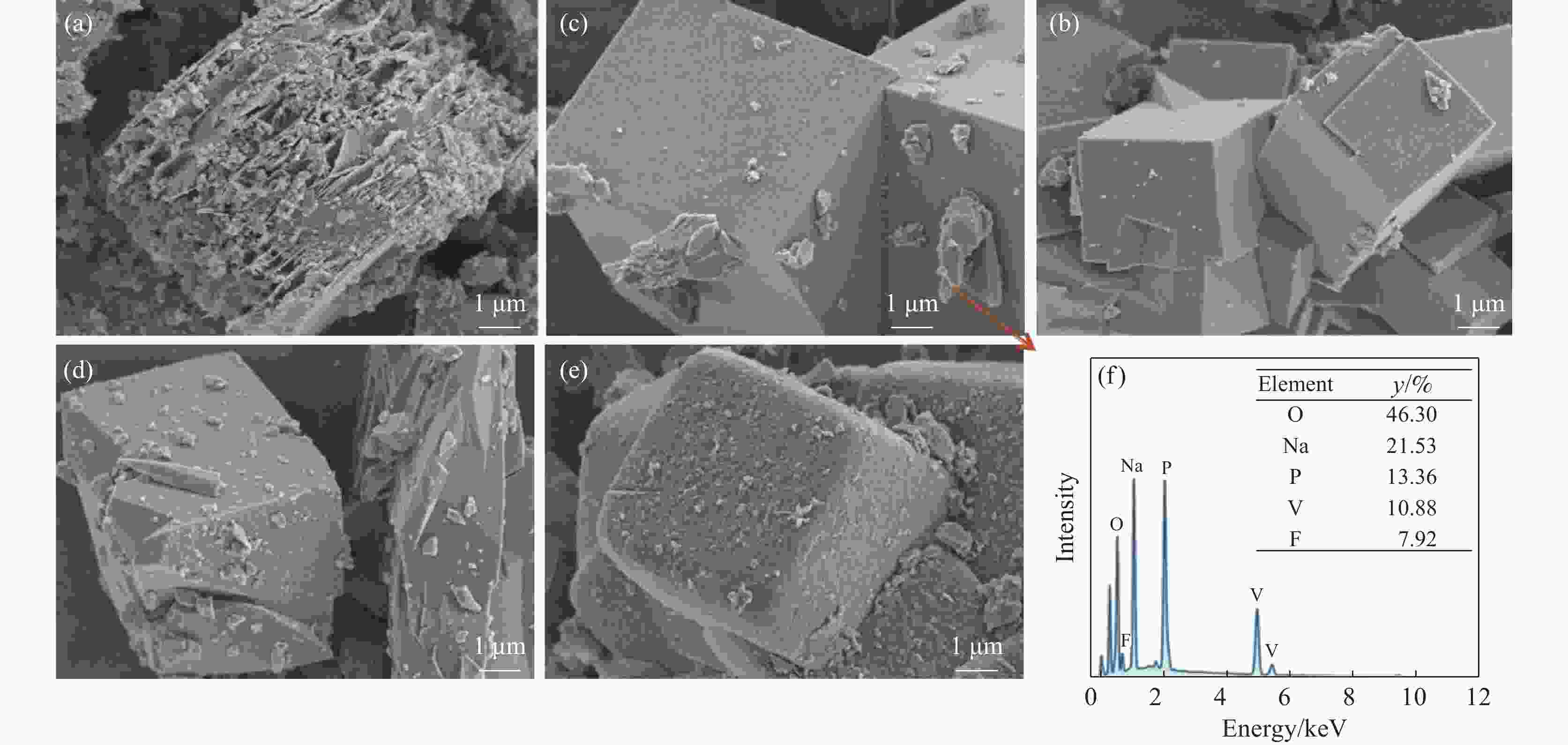
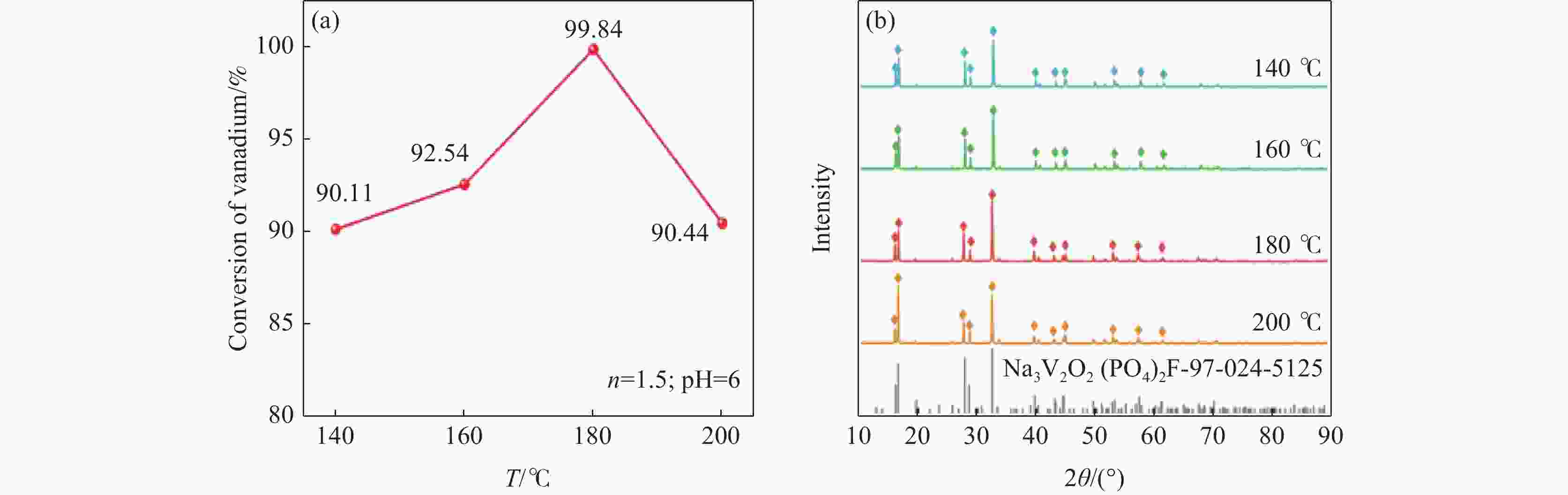
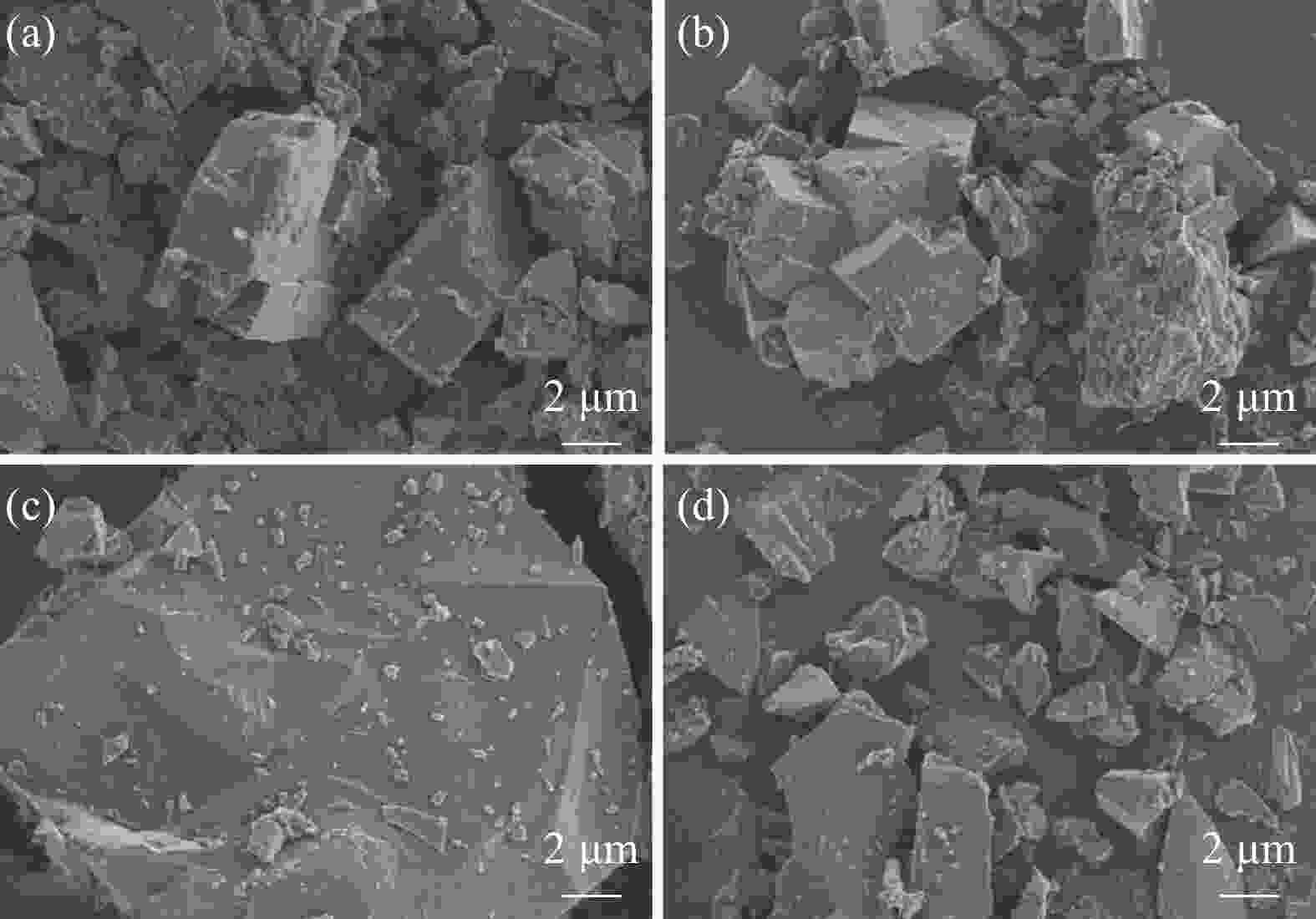
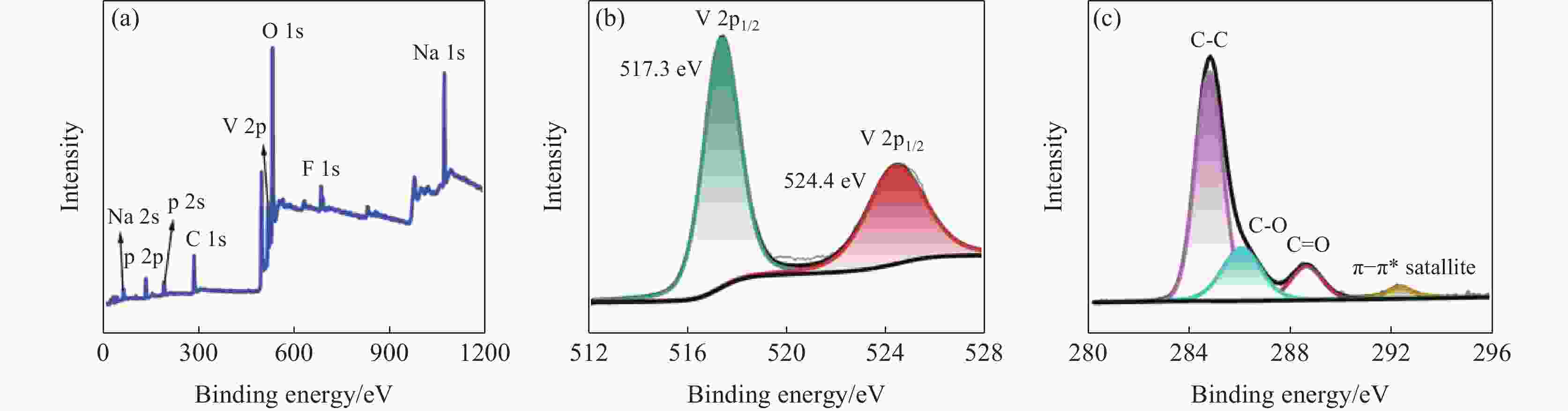
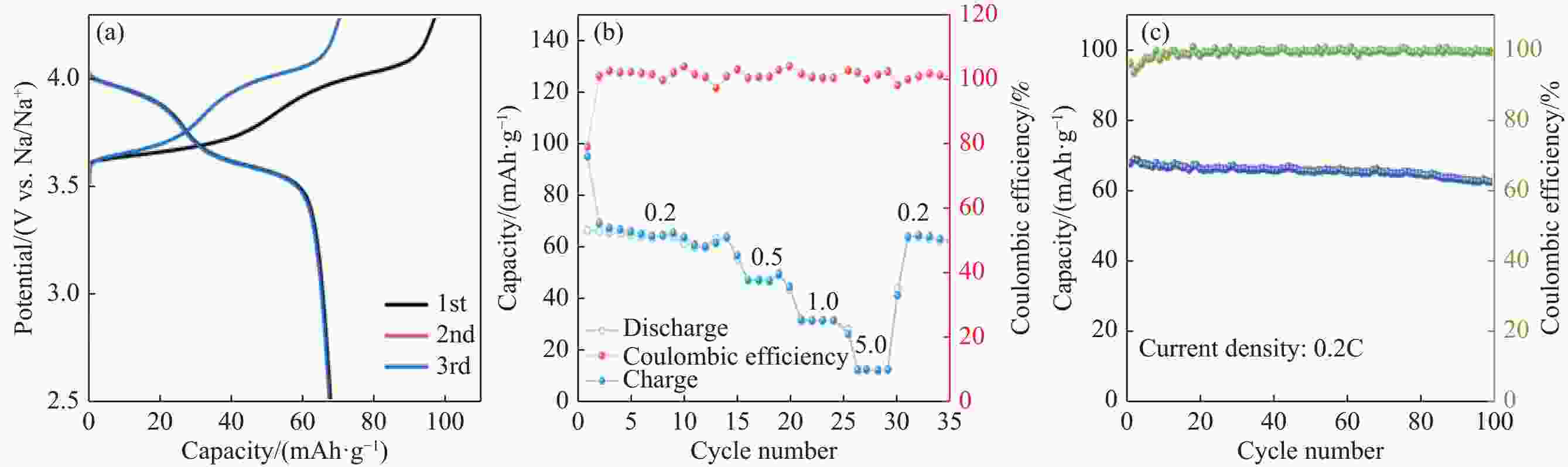
摘要: 针对传统氟磷酸钒钠(NVOPF)制备过程中使用钒源的高成本困境,提出了一种新型溶剂热合成工艺,利用钠化钒渣水浸液替代高纯钒源,成功实现了从冶金副产品到电池材料的直接转化。此外,探讨了还原剂柠檬酸 (C6H8O7) 用量、溶液pH值和合成温度对钒转化率及材料性能的影响。试验结果表明,随着还原剂用量、pH值和温度的增加,钒转化率呈现先升后降的趋势。在溶液pH值为6、柠檬酸与钒摩尔比为1.5、溶剂热温度为180 ℃条件下,合成的NVOPF材料呈现规则的立方块状结构,钒为正四价 (V4+),在0.2C倍率下的首次放电容量为68 mAh/g,表现出良好的结构稳定性,第三圈库伦效率为96%。该材料的高倍率性能和实际容量与理论值之间仍存在一定差距,主要受浸出液杂质的影响。此项研究为钒渣的高附加值利用及低成本钠离子电池正极材料的制备提供了新的解决思路。Abstract: To address the dilemma of high-cost in using vanadium sources for the traditional preparation of sodium vanadium fluorophosphate (NVOPF), a novel solvothermal synthesis process was proposed. This method utilizes sodium-treated vanadium slag leaching solution as a substitute for high-purity vanadium sources, and successfully realized the direct conversion from metallurgical by-products to battery materials. In addition, the effects of the dosage of reducing agent citric acid (C6H8O7), solution pH, and synthesis temperature on vanadium conversion rate and material properties were investigated. The experimental results showed that with the increase of the dosage of reducing agent, pH, and temperature, the vanadium conversion rate showed a trend of first increasing and then decreasing. Under the conditions of pH value of solution of 6, molar ratio of citric acid to vanadium of 1.5, and solvothermal temperature of 180 °C, the synthesized NVOPF material exhibited a regular cubic block structure with vanadium in the +4 oxidation state (V4+) and the initial discharge capacity at a 0.2C rate was 68 mAh/g, demonstrating good structural stability and a third-cycle Coulombic efficiency of 96%. However, there is still a certain gap between the high-rate performance and actual capacity of the material and the theoretical value, which is primarily affected by the impurities in the leaching solution. This study provides a new solution for the high value added utilization of vanadium slag and the preparation of low-cost cathode materials for sodium-ion battery.
-
表 1 含钒浸出液中主要离子的浓度
Table 1. Concentration of main ions in vanadium leaching solution
g/L V5+ Na+ Si2+ Ca2+ Cr3+ 15.08 13.85 0.57 0.27 0.30 表 2 NVOPF产物纯度及其杂质含量
Table 2. Purity of NVOPF products and content of impurities
% NVOPF Cr2O3 CaO SiO2 Bal. 99.50 0.24 0.05 0.10 0.11 -
[1] LEE J C, KURNIAWAN, KIM E Y, et al. A review on the metallurgical recycling of vanadium from slags: towards a sustainable vanadium production[J]. Journal of Materials Research and Technology, 2021, 12: 343-364. doi: 10.1016/j.jmrt.2021.02.065 [2] GUO Y, LI H Y, SHEN S, et al. Recovery of vanadium from vanadium slag with high phosphorus content via recyclable microemulsion extraction[J]. Hydrometallurgy, 2020, 198: 105509. doi: 10.1016/j.hydromet.2020.105509 [3] WEN J, JIANG T, XU Y, et al. Efficient extraction and separation of vanadium and chromium in high chromium vanadium slag by sodium salt roasting-(NH4)2SO4 leaching[J]. Journal of Industrial and Engineering Chemistry, 2019, 71: 327-335. doi: 10.1016/j.jiec.2018.11.043 [4] ELLIS B L, NAZAR L F. Sodium and sodium-ion energy storage batteries[J]. Current Opinion in Solid State and Materials Science, 2012, 16(4): 168-177. doi: 10.1016/j.cossms.2012.04.002 [5] LENG M, BI J, et al. Superior electrochemical performance of O3-type NaNi0.5-xMn0.3Ti0.2ZrxO2 cathode material for sodium-ion batteries from Ti and Zr substitution of the transition metals[J]. Journal of Alloys and Compounds, 2020, 816: 152581. doi: 10.1016/j.jallcom.2019.152581 [6] ZHENG L, ZHANG D, et al. Continuous-flow rapid and controllable microfluidic synthesis of sodium vanadium fluorophosphate as a cathode material[J]. Applied Materials Today, 2021, 23: 101032. doi: 10.1016/j.apmt.2021.101032 [7] HUANG X, ZHANG K, LIANG F, et al. Optimized solvothermal synthesis of LiFePO4 cathode material for enhanced high-rate and low temperature electrochemical performances[J]. Electrochimica Acta, 2017, 258: 1149-1159. doi: 10.1016/j.electacta.2017.11.167 [8] JING Q, ZHANG J, YANG C, et al. A novel and practical hydrothermal method for synthesizing LiNi1/3Co1/3Mn1/3O2 cathode material[J]. Ceramics International, 2020, 46(12): 20020-20026. doi: 10.1016/j.ceramint.2020.05.073 [9] GOVER R, BRYAN A, BURNS P, et al. The electrochemical insertion properties of sodium vanadium fluorophosphate, Na3V2(PO4)2F3[J]. Solid State Ionics, 2006, 177(17-18): 1495-1500. doi: 10.1016/j.ssi.2006.07.028 [10] LI Z Y, TANG T, WANG Z H, et al. Preparation of lithium manganese iron phosphate cathode material from vanadium tailings[J]. Iron Steel Vanadium Titanium, 2024, 45(6): 19-27. (李智宇, 汤婷, 王正豪, 等. 从提钒尾液制备磷酸锰铁锂正极材料的研究[J]. 钢铁钒钛, 2024, 45(6): 19-27. doi: 10.7513/j.issn.1004-7638.2024.06.003LI Z Y, TANG T, WANG Z H, et al. Preparation of lithium manganese iron phosphate cathode material from vanadium tailings[J]. Iron Steel Vanadium Titanium, 2024, 45(6): 19-27. doi: 10.7513/j.issn.1004-7638.2024.06.003 [11] WANG S W, ZHENG H, WANG J P. A method for directly preparing sodium vanadium phosphate cathode material from sodium-based vanadium solution: CN117361485A[P]. 2024-01-09. (王仕伟, 郑浩, 汪劲鹏. 一种钠法钒液直接制备磷酸钒钠正极材料的方法: 117361485A[P]. 2024-01-09.WANG S W, ZHENG H, WANG J P. A method for directly preparing sodium vanadium phosphate cathode material from sodium-based vanadium solution: CN117361485A[P]. 2024-01-09. [12] NI W, XIN Y N, YANG Y, et al. A carbon-composite sodium vanadium phosphate-based cathode material for sodium-ion batteries and its short-process preparation method: CN119409157A[P]. 2025-02-11. (倪伟, 辛亚男, 杨亚, 等. 一种碳复合磷酸钒钠基钠电正极材料及其短流程制备方法: 119409157A[P]. 2025-02-11.NI W, XIN Y N, YANG Y, et al. A carbon-composite sodium vanadium phosphate-based cathode material for sodium-ion batteries and its short-process preparation method: CN119409157A[P]. 2025-02-11. [13] MOSER T. Stability of model V P O catalysts for maleic anhydride synthesis[J]. Journal of Catalysis, 1987, 104(1): 99-108. doi: 10.1016/0021-9517(87)90340-X [14] XIE L, YANG Y, FU Z, et al. Fe/Zn-modified tricalcium phosphate (TCP) biomaterials: preparation and biological properties[J]. RSC Advances, 2019, 9(2): 781-789. doi: 10.1039/C8RA08453J [15] SHI G K, XIE J P, LI Z B, et al. A bismuth oxide-modified copper host achieving bubble-free and stable potassium metal batteries[J]. Chemical Science, 2025, 16(3): 1344-1352. doi: 10.1039/D4SC07483A [16] IGARASHI H, TSUJI K, OKUHARA T, et al. Effects of consecutive oxidation on the production of maleic anhydride in butane oxidation over four kinds of well-characterized vanadyl pyrophosphates[J]. Journal of Physical Chemistry, 1993, 97(27): 7065-7071. doi: 10.1021/j100129a023 [17] PHAM Q N, WINTER M, MILANOVA V, et al. Magnetic enrichment of immuno-specific extracellular vesicles for mass spectrometry using biofilm-derived iron oxide nanowires[J]. Bioengineering, 2022. [18] LUO Q C, ZHANG D B, YUAN X R, et al. Revealing the regulation mechanism of Na3V2(PO4)2O2F crystal growth in sodium alginate solution for high-performance sodium ion batteries[J]. Journal of Power Sources, 2024, 623: 235438. doi: 10.1016/j.jpowsour.2024.235438 [19] ZHANG D B, YUAN X R, XIN Y N, et al. Research on preparation of nano sodium vanadium phosphate and its sodium storage properties[J]. Iron Steel Vanadium Titanium, 2024, 45(1): 12-18. (张东彬, 袁欣然, 辛亚男, 等. 纳米磷酸钒钠的制备及其储钠性能研究[J]. 钢铁钒钛, 2024, 45(1): 12-18. doi: 10.7513/j.issn.1004-7638.2024.01.003ZHANG D B, YUAN X R, XIN Y N, et al. Research on preparation of nano sodium vanadium phosphate and its sodium storage properties[J]. Iron Steel Vanadium Titanium, 2024, 45(1): 12-18. doi: 10.7513/j.issn.1004-7638.2024.01.003 [20] NI W. Low-dimensional vanadium-based high-voltage cathode materials for promising rechargeable alkali-ion batteries[J]. Materials, 2024, 17: 587. doi: 10.3390/ma17030587 -




 下载:
下载: