Preparation of lithium manganese iron phosphate cathode material by purification of ferrous sulfate and study on its performance influence
-
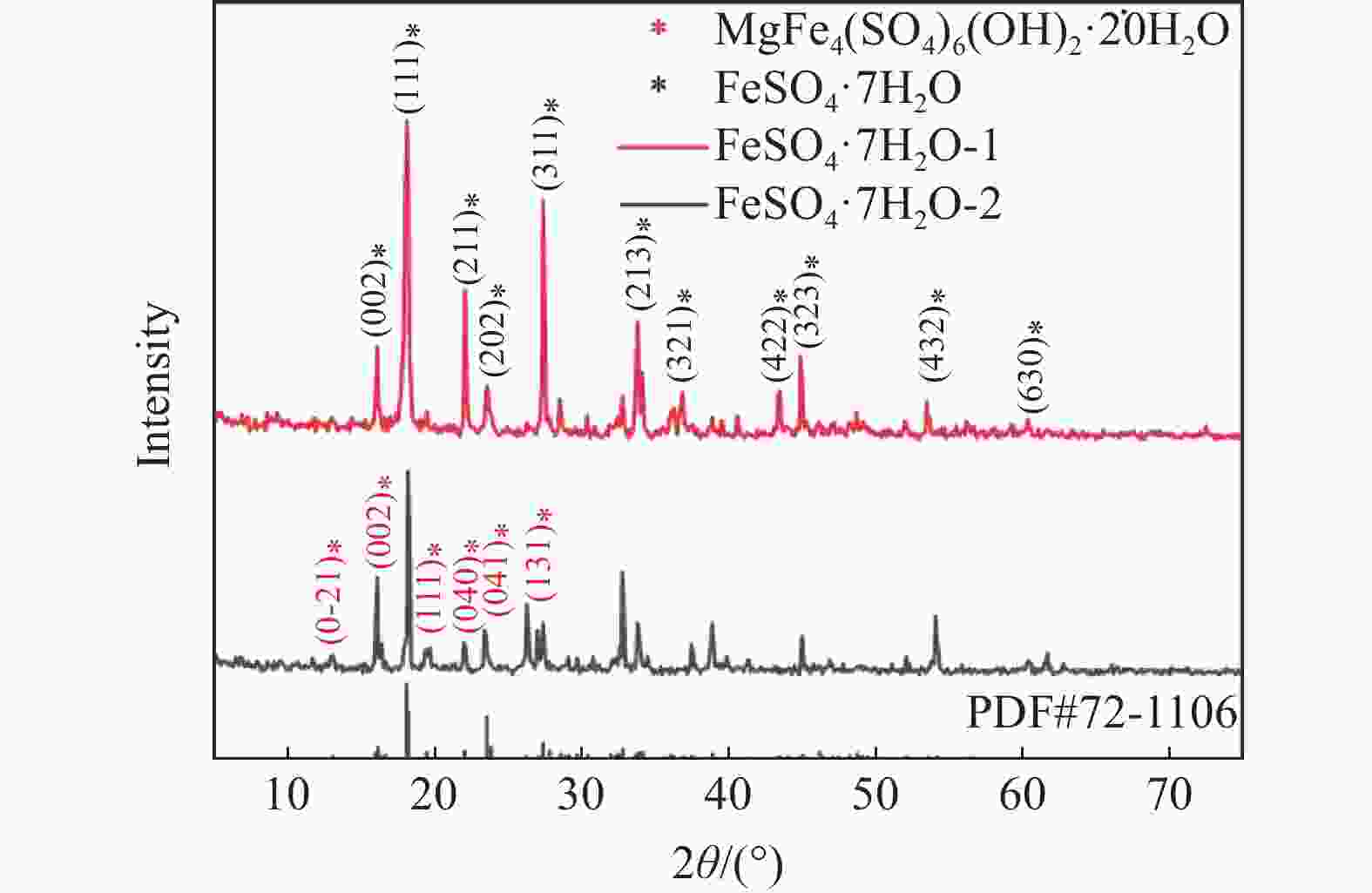
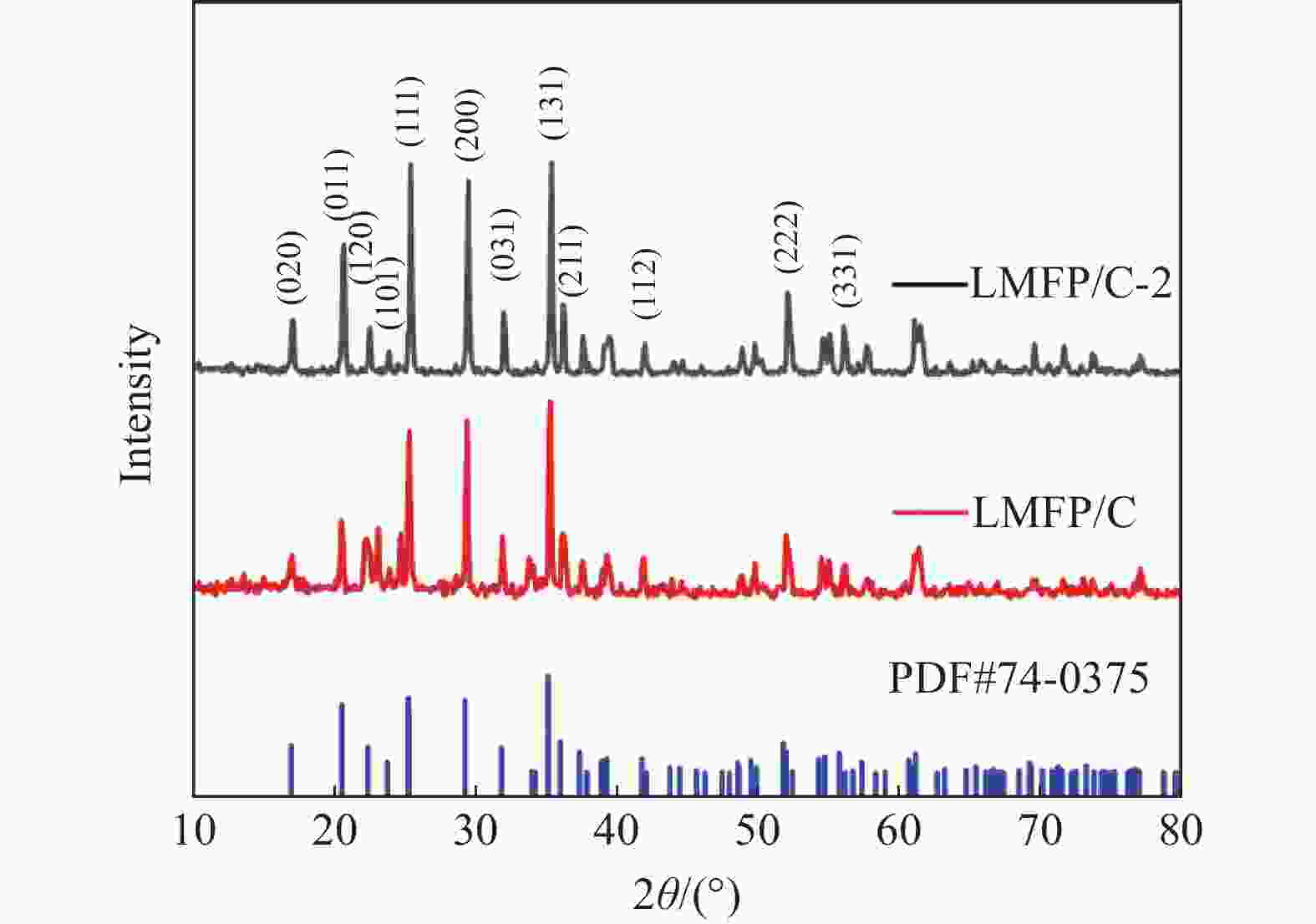
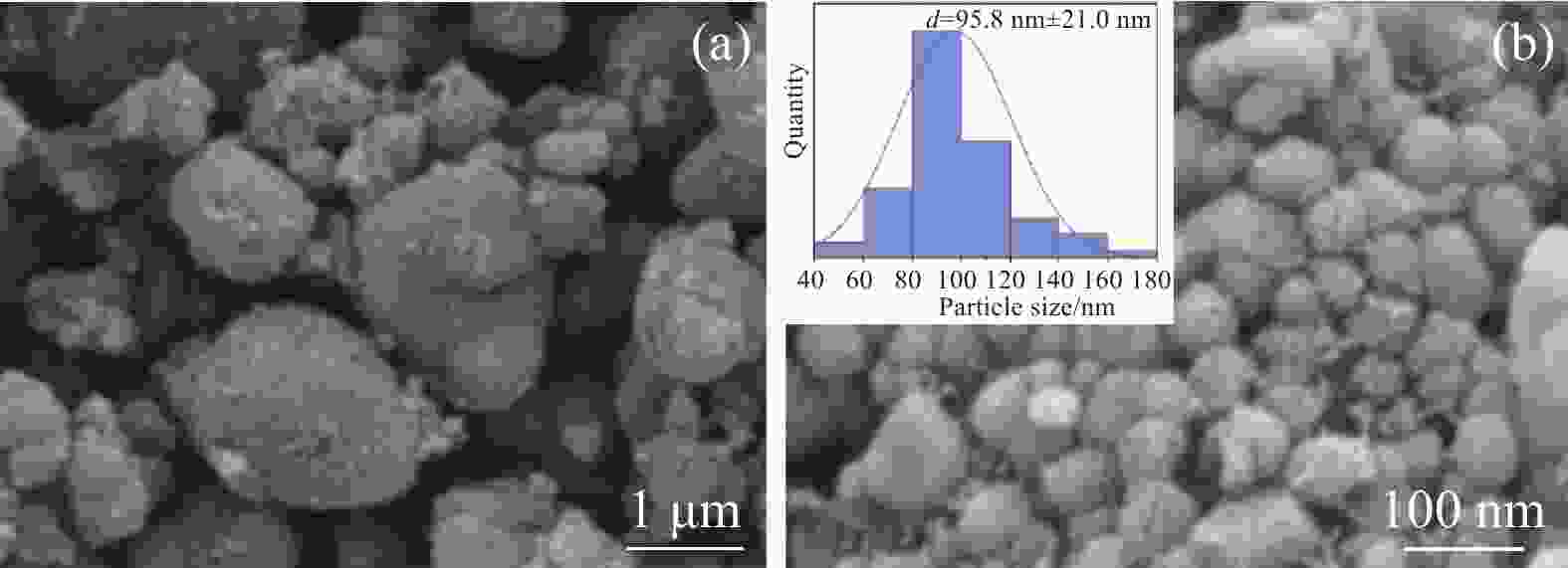
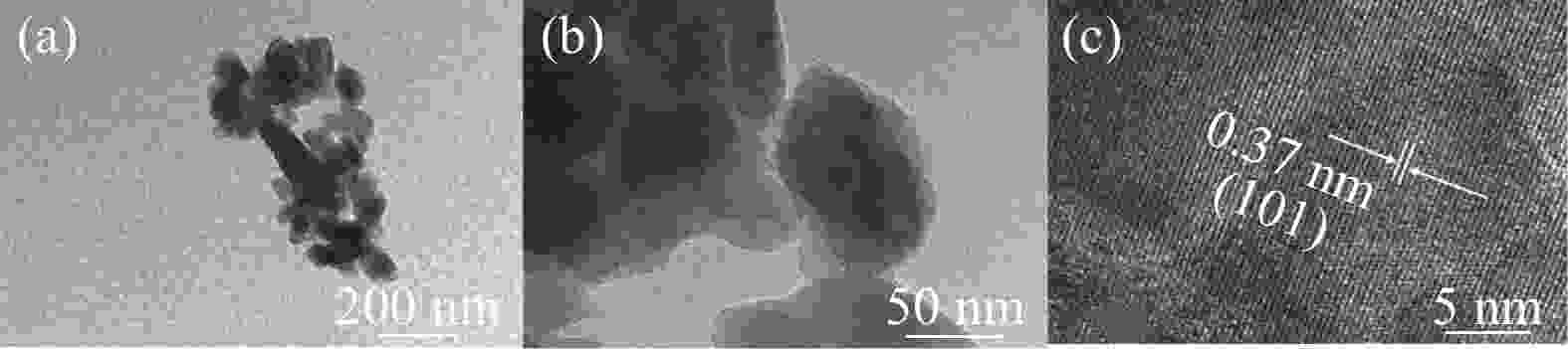
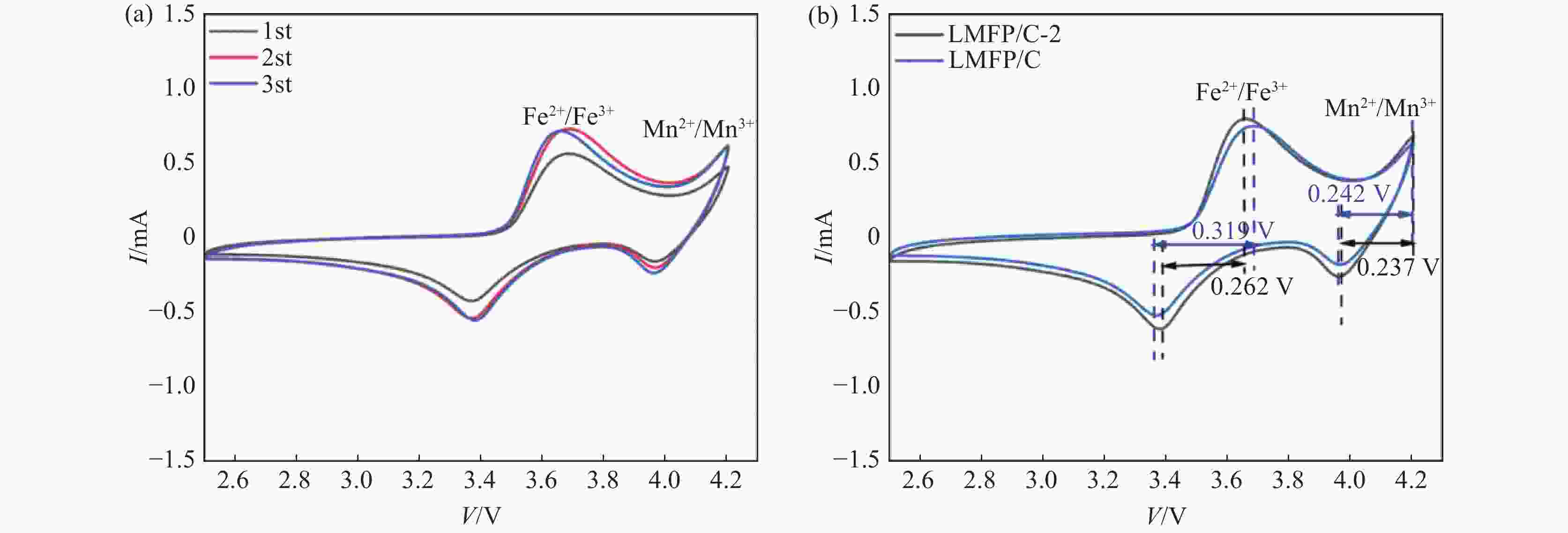
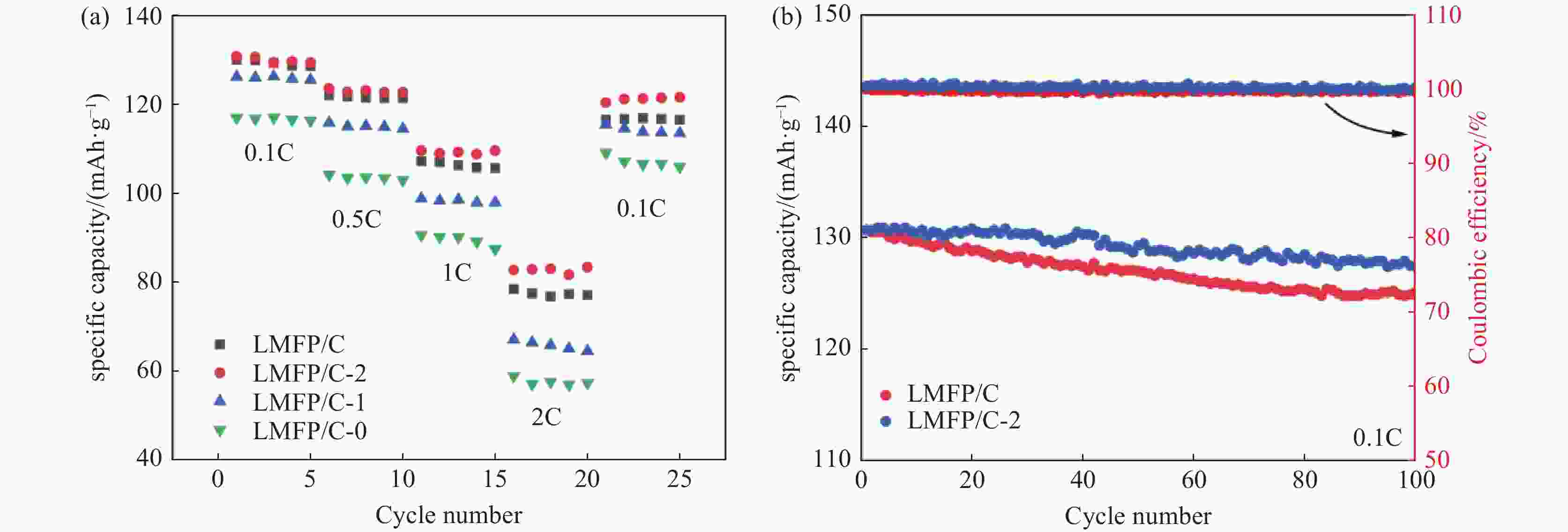
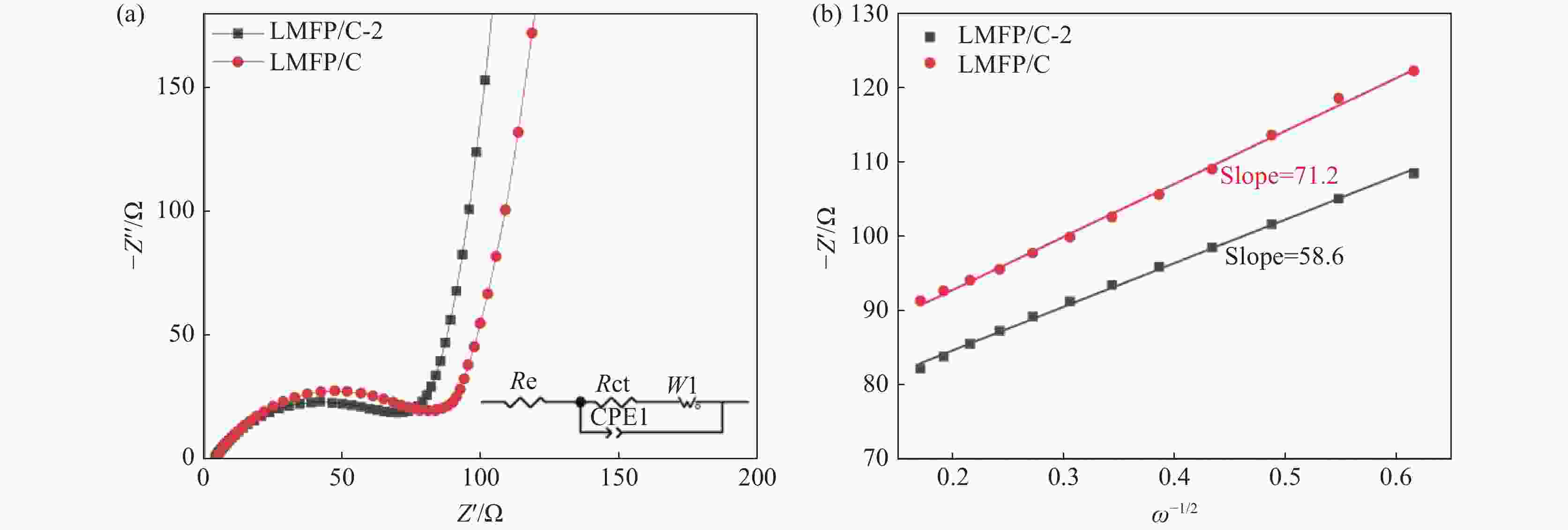
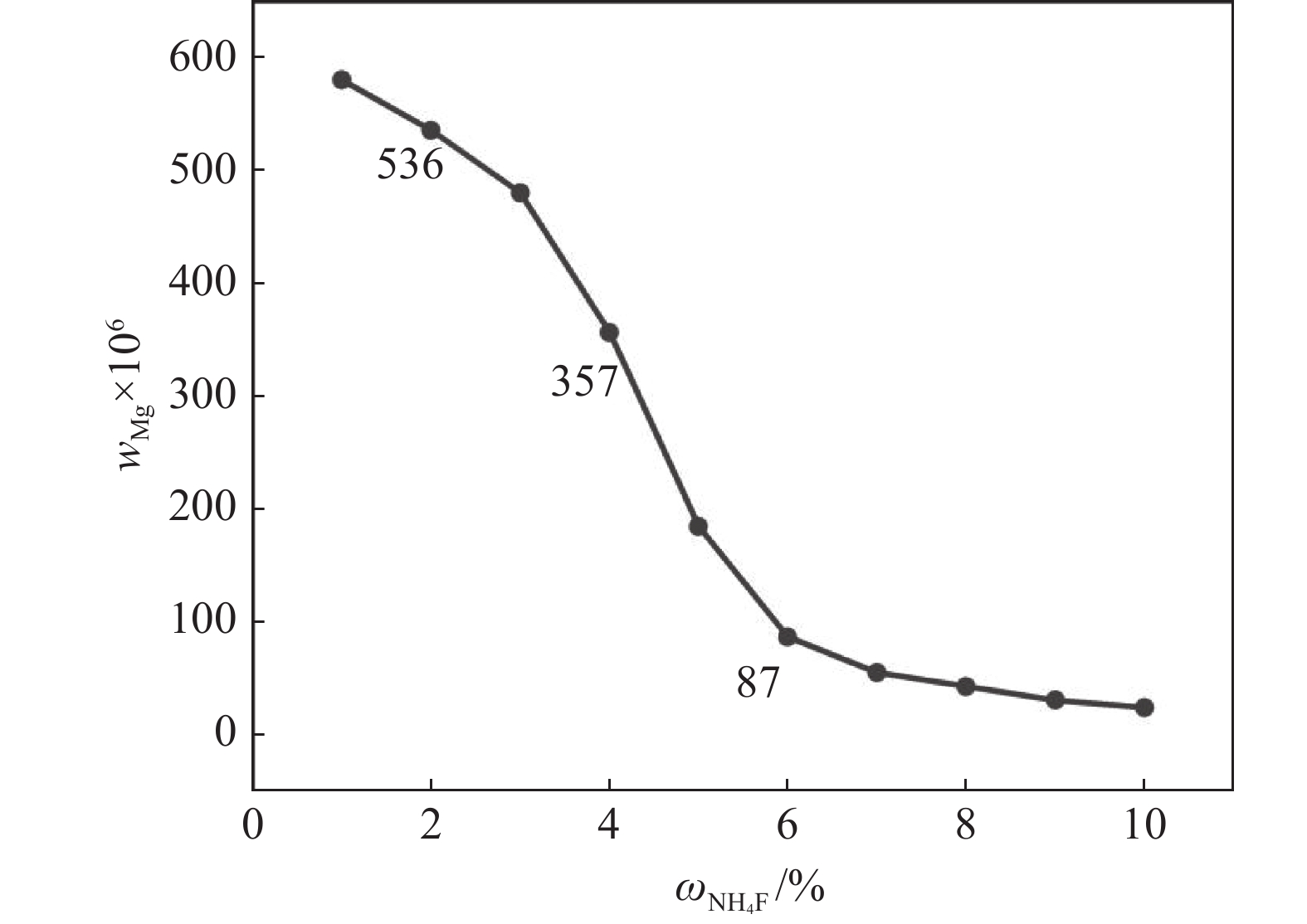
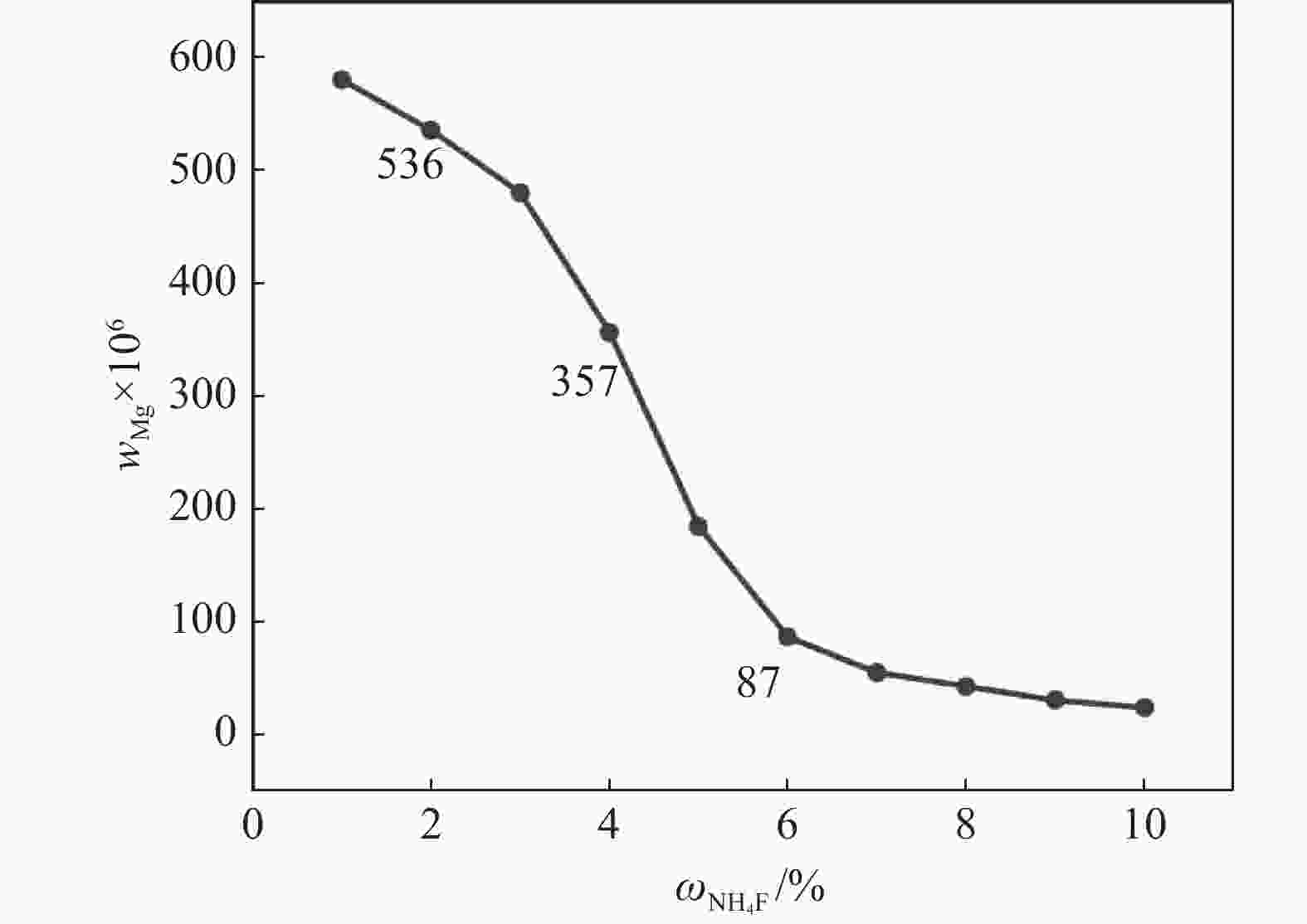
摘要: 采用硫酸法工艺在生产钛白粉的过程中产生大量的副产品硫酸亚铁,以提纯后硫酸亚铁作为原料,采用一步水热法合成经济价值更高的磷酸锰铁锂正极材料,研究原料中部分去除的镁元素对磷酸锰铁锂正极材料的物理和电化学性能的影响。结果显示,原料采用质量分数为6%的氟化铵为化学沉淀剂,得到镁杂质脱除率为98.86%的硫酸亚铁产品,合成的材料为不规则球形形貌正交晶系的磷酸锰铁锂材料,少量镁杂质改变了材料中锂离子的活动空间,使锂离子迁移速率得到提升,合成的磷酸锰铁锂正极材料(LMFP/C-2)放电比容量在0.1C和2C下分别为135.24 mAh/g和86.16 mAh/g,在0.1C下循化100圈后放电比容量保持率可达到97.70%,所得产物稍优于高纯度商业材料的性能。Abstract: The titanium dioxide production process via the sulfuric acid method generates a significant amount of byproduct ferrous sulfate. To fully utilize this resource, ferrous sulfate is purified and then used as a raw material to synthesize value-added cathode material of lithium manganese iron phosphate (LMFP) through a one-step hydrothermal method. In this study the impact of partially removed magnesium from the raw material on the physical and electrochemical properties of LMFP had been investigated. The results show that the using ammonium fluoride with a mass fraction of 6% as a chemical precipitant can obtain ferrous sulfate products with a removal rate of 98.86% magnesium impurities. Consequently the synthesized LMFP features an irregular spherical morphology and an orthorhombic crystal structure. A small amount of magnesium impurities alters the lithium-ion activity space within the material, enhancing lithium-ion migration rates. The discharge specific capacity of the synthesized LMFP cathode material (LMFP/C-2) is 135.24 mAh/g at 0.1C and 86.16 mAh/g at 2C, respectively. After 100 cycles at 0.1C, the discharge specific capacity retention rate reaches 97.70%. The performance of the obtained product slightly surpasses that of high-purity commercial materials.
-
表 1 七水硫酸亚铁主要化学成分
Table 1. Main chemical composition of ferrous sulfate
% Fe Ti S Mg Ca Al Zn 26 0.03 15 0.76 0. 046 0.002 0.008 注:其余为游离水 表 2 七水硫酸亚铁提纯后各元素质量分数
Table 2. Mass fraction of each element in the product after purifying ferrous sulfate heptahydrate
% Fe Ti Mn Mg Ca Al Zn 99.97 0.003 0.004 0.0087 0. 001 0.0015 0.008 注:其余为游离水 表 3 LMFP/C与LMFP/C-2样品的晶胞参数与晶胞体积
Table 3. Cell parameters and cell volumes of LMFP/C and LMFP/C-2 samples
项目 a/nm b/nm c/nm V/nm3 LMFP/C 1.046 0.604 0.474 29.949 LMFP/C-2 1.041 0.606 0.474 29.863 -
[1] WU Y F, BAI L F, WANG P F, et al. Research on positive electrode materials for lithium ion batteries[J]. Power Technology, 2019, 43(9): 1547-1550. (吴怡芳, 白利锋, 王鹏飞, 等. 锂离子电池正极材料研究[J]. 电源技术, 2019, 43(9): 1547-1550. doi: 10.3969/j.issn.1002-087X.2019.09.038WU Y F, BAI L F, WANG P F, et al. Research on positive electrode materials for lithium ion batteries[J]. Power Technology, 2019, 43(9): 1547-1550. doi: 10.3969/j.issn.1002-087X.2019.09.038 [2] RAO Y Y, WANG K P, ZENG H. Research progress of lithium manganese iron phosphate materials in lithium batteries[J]. Power Technology, 2016, 40(2): 455-457. (饶媛媛, 王康平, 曾晖. 磷酸锰铁锂材料在锂电池中的研究进展[J]. 电源技术, 2016, 40(2): 455-457. doi: 10.3969/j.issn.1002-087X.2016.02.067RAO Y Y, WANG K P, ZENG H. Research progress of lithium manganese iron phosphate materials in lithium batteries[J]. Power Technology, 2016, 40(2): 455-457. doi: 10.3969/j.issn.1002-087X.2016.02.067 [3] DU H, KANG Y, LI C, et al. Easily recyclable lithium-ion batteries: Recycling-oriented cathode design using highly soluble LiFeMnPO4 with a water-soluble binder[J]. Battery Energy, 2023, 2(4): 20230011. doi: 10.1002/bte2.20230011 [4] SU B C, ZHANG Q, XIE Y J, et al. Research progress on synthesis methods and structural modification of lithium iron manganese phosphate materials[J]. Inorganic Salt Industry, 2024, 56(7): 28-36. (苏宝才, 张勤, 谢元健, 等. 磷酸铁锰锂材料的合成方法及结构改性的研究进展[J]. 无机盐工业, 2024, 56(7): 28-36.SU B C, ZHANG Q, XIE Y J, et al. Research progress on synthesis methods and structural modification of lithium iron manganese phosphate materials[J]. Inorganic Salt Industry, 2024, 56(7): 28-36. [5] WANG L, LI Y, WU J, et al. Synthesis mechanism and characterization of LiMn0.5Fe0.5PO4/C composite cathode material for lithium-ion batteries[J]. Journal of Alloys and Compounds, 2020, 839: 155653. doi: 10.1016/j.jallcom.2020.155653 [6] LUO T, ZENG T, CHEN S, et al. Structure, performance, morphology and component transformation mechanism of LiMn0.8Fe0.2PO4/C nanocrystal with excellent stability[J]. Journal of Alloys and Compounds, 2020, 834: 155143. doi: 10.1016/j.jallcom.2020.155143 [7] WANG Y, HU G, CAO Y, et al. Highly atom-economical and environmentally friendly synthesis of LiMn0.8Fe0.2PO4/rGO/C cathode material for lithium-ion batteries[J]. Electrochimica Acta, 2020, 354: 136743. doi: 10.1016/j.electacta.2020.136743 [8] BEZZA I, AZIAM H, SAADOUNE I. On the LiFe1-xMnxPO4 (x= 0, 0.4, 0.6, 0.65, 1) olivine-type cathode materials for lithium ion batteries[J]. Materials Today: Proceedings, 2022, 51: 1913-1917. doi: 10.1016/j.matpr.2021.02.648 [9] BI S. The current situation, future and development of China's titanium dioxide industry in 2023[J]. Iron Steel Vanadium Titanium, 2024, 45(1): 1-3. (毕胜. 2023年中国钛白粉行业的现状、未来及发展[J]. 钢铁钒钛, 2024, 45(1): 1-3. doi: 10.7513/j.issn.1004-7638.2024.01.001BI S. The current situation, future and development of China's titanium dioxide industry in 2023[J]. Iron Steel Vanadium Titanium, 2024, 45(1): 1-3. doi: 10.7513/j.issn.1004-7638.2024.01.001 [10] CHEN P, ZHENG X, CHENG W. Biochar combined with ferrous sulfate reduces nitrogen and carbon losses during agricultural waste composting and enhances microbial diversity[J]. Process Safety and Environmental Protection, 2022, 162: 531-542. doi: 10.1016/j.psep.2022.04.042 [11] GAO G Y, GAO L K, RAO B, et al. The current status and prospects of resource utilization of sulfuric acid titanium dioxide waste acid[J]. Iron Steel Vanadium Titanium, 2021, 42(5): 99-108. (高广言, 高利坤, 饶兵, 等. 硫酸法钛白废酸资源化利用现状及展望[J]. 钢铁钒钛, 2021, 42(5): 99-108. doi: 10.7513/j.issn.1004-7638.2021.05.016GAO G Y, GAO L K, RAO B, et al. The current status and prospects of resource utilization of sulfuric acid titanium dioxide waste acid[J]. Iron Steel Vanadium Titanium, 2021, 42(5): 99-108. doi: 10.7513/j.issn.1004-7638.2021.05.016 [12] GUO J. Research on the process technology of purifying titanium white slag to prepare battery grade ferrous sulfate[J]. Inorganic Salt Industry, 2019, 51(8): 48-51. (郭举. 钛白渣提纯制备电池级硫酸亚铁工艺技术研究[J]. 无机盐工业, 2019, 51(8): 48-51. doi: 10.11962/1006-4990.2018-0675GUO J. Research on the process technology of purifying titanium white slag to prepare battery grade ferrous sulfate[J]. Inorganic Salt Industry, 2019, 51(8): 48-51. doi: 10.11962/1006-4990.2018-0675 [13] YUAN W L, WANG B X, ZHAO Y, et al. Synthesis of iron phosphate precursor from ferrous sulfate, a byproduct of titanium dioxide[J]. Nonferrous Metals Engineering, 2023, 13(7): 61-68. (袁文龙, 王碧侠, 赵瑛, 等. 用钛白副产硫酸亚铁合成磷酸铁前驱体[J]. 有色金属工程, 2023, 13(7): 61-68. doi: 10.3969/j.issn.2095-1744.2023.07.009YUAN W L, WANG B X, ZHAO Y, et al. Synthesis of iron phosphate precursor from ferrous sulfate, a byproduct of titanium dioxide[J]. Nonferrous Metals Engineering, 2023, 13(7): 61-68. doi: 10.3969/j.issn.2095-1744.2023.07.009 [14] WEN Z P, PAN K, WEI Y, et al. Research progress on modification of lithium manganese iron phosphate cathode materials[J]. Energy Storage Science and Technology, 2024, 13(3): 770-787. (文志朋, 潘凯, 韦毅, 等. 磷酸锰铁锂正极材料改性研究进展[J]. 储能科学与技术, 2024, 13(3): 770-787.WEN Z P, PAN K, WEI Y, et al. Research progress on modification of lithium manganese iron phosphate cathode materials[J]. Energy Storage Science and Technology, 2024, 13(3): 770-787. [15] JANG D, PALANISAMY K, KIM Y, et al. Structural and electrochemical properties of doped LiFe0.48Mn0.48Mg0.04PO4 as cathode material for lithium ion batteries[J]. Journal of Electrochemical Science and Technology, 2013, 4(3): 102-107. doi: 10.33961/JECST.2013.4.3.102 [16] ZHANG X, HOU M, TAMIRATE A G, et al. Carbon coated nano-sized LiMn0.8Fe0.2PO4 porous microsphere cathode material for Li-ion batteries[J]. Journal of Power Sources, 2020, 448: 227438. doi: 10.1016/j.jpowsour.2019.227438 [17] JIN H, ZHANG J, QIN L, et al. Dual modification of olivine LiFe0. 5Mn0. 5PO4 cathodes with accelerated kinetics for high-rate lithium-ion batteries[J]. Industrial & Engineering Chemistry Research, 2023, 62(2): 1029-1034. [18] LI C W, XU S G, YU H F, et al. Research on magnesium doped modified LiMn0. 5Fe0. 5PO4/C positive electrode material and properties[J]. Energy Storage Science and Technology, 2024, 13(6): 1767-1774. (李晨威, 徐世国, 余海峰, 等. 镁掺杂改性LiMn0.5Fe0.5PO4/C正极材料与性能研究[J]. 储能科学与技术, 2024, 13(6): 1767-1774.LI C W, XU S G, YU H F, et al. Research on magnesium doped modified LiMn0. 5Fe0. 5PO4/C positive electrode material and properties[J]. Energy Storage Science and Technology, 2024, 13(6): 1767-1774. [19] LUO S, SUN Y, BAO S, et al. Synthesis of Er-doped LiMnPO4/C by a sol-assisted hydrothermal process with superior rate capability[J]. Journal of Electroanalytical Chemistry, 2019, 832: 196-203. doi: 10.1016/j.jelechem.2018.10.062 [20] XU W, ZHOU Y, JI X. Lithium-ion-transfer kinetics of single LiFePO4 particles[J]. The Journal of Physical Chemistry Letters, 2018, 9(17): 4976-4980. doi: 10.1021/acs.jpclett.8b02315 -




 下载:
下载: