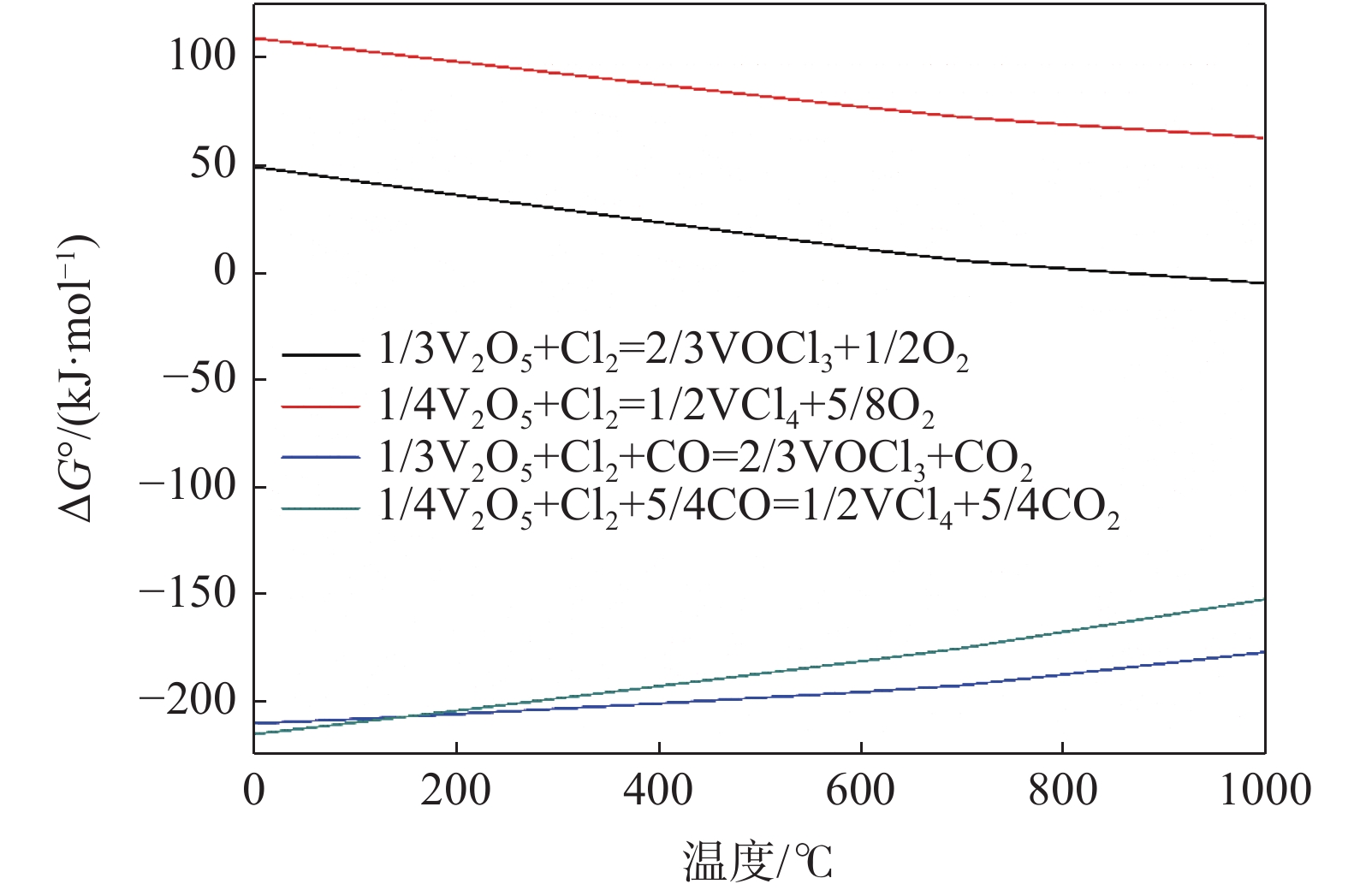
Review on research progress of high purity vanadium pentoxide preparation by chlorination process
-
摘要: 随着全钒液流电池等产业的快速发展,作为关键原料的高纯五氧化二钒,其需求日益增加。在高纯五氧化二钒的众多制备工艺中,氯化法因具有高效清洁、选择性高、产品纯度高等显著优势受到越来越多的关注。针对氯化法制备高纯五氧化二钒的三个关键技术环节:钒原料氯化过程的热力学、动力学和氯化工艺、粗三氯氧钒精制以及三氯氧钒转化制备五氧化二钒的研究进展结合相关文献进行系统评述,其中二次钒资源的高效利用,粗三氯氧钒选择性水解四氯化钛以及三氯氧钒催化氧化制备高纯五氧化二钒是各环节未来的发展方向,此外,开展氯化法制备高纯五氧化二钒的中间试验及关键装备技术的开发是未来实现产业化生产的重点及难点。Abstract: As a crucial raw material for all-vanadium redox flow battery(VRFB), the high purity vanadium pentoxide (V2O5) is under an increasingly urgent demand with the rapid development of VRFB in recent years. Based on this background, a plenty of processes have been proposed for high purity V2O5 preparation, among which the chlorination process is paid more and more attentions due to the remarkable advantages of high efficiency, favorable selectivity, high purity of products and eco-friendly feature. In this paper, we reviewed the research progress with relevant literatures concentrated on the three key procedures of high purity V2O5 preparation via chlorination process, including thermodynamics, kinetics and processes of chlorination of raw materials, purification of crude vanadium oxytrichloride (VOCl3) and conversion of VOCl3 into V2O5. The high-efficient utilization of secondary vanadium resources, the selective hydrolysis of crude vanadium oxychloride to remove TiCl4 and conversion of VOCl3 into V2O5 by catalytic oxidation are proposed as the development trends of the corresponding key procedures. Finally, carrying out the pilot test and developing the key equipment for preparing high-purity vanadium pentoxide by chlorination process are the key and difficult points for realizing the industrial production in the future.
-
表 1 不同氯化体系动力学参数
Table 1. Kinetics parameters of different chlorination systems
氯化体系 反应级数 温度/℃ 活化能/(kJ·mol−1) 控制步骤 Cl2+N2 0.78 <570 235 化学反应 570<T<650 77 化学反应,内扩散 Cl2+CO+N2 <650 100 化学反应 >680 105 化学反应 CCl4 0.5 77±5 化学反应 表 2 不同钒原料氯化反应条件及钒的氯化率
Table 2. Parameters and vanadium extraction ratio for chlorination of different vanadium raw materials
含钒原料 氯化剂 反应器类型 氯化温度/℃ 时间/h 钒氯化率/% 反应方程 工业级五氧化二钒 AlCl3 固定床 170 2 83 1/2V2O5+AlCl3→VOCl3+1/2Al2O3 钒渣 Cl2 流化床 650 2 87 1/5Mn2V2O7+Cl2+1/2C→2/5MnCl2+2/5VOCl3+1/2CO2 含钒废催化剂 Cl2 固定床 400~550 0.5 73 1/3V2O5+Cl2→2/3VOCl3+1/2O2 钒钛磁铁矿 FeCl2 固定床 827 2 22 1/2V2O5+FeCl3→ VOCl3+1/2Fe2O3 FeCl3 827 2 32 1/3V2O5+FeCl2+1/4O2→ 2/3VOCl3+1/2Fe2O3 石煤 Cl2 固定床 1000 1 90 1/3V2O3+Cl2→2/3VOCl3+1/6O2 注:钒渣预处理工艺为900 ℃预氧化2 h;钒钛磁铁矿预处理工艺为900 ℃预氧化2 h;石煤预处理工艺为600 ℃预脱碳2 h。 -
[1] Peng H. A literature review on leaching and recovery of vanadium[J]. Journal of Environmental Chemical Engineering, 2019,7(5):103313. doi: 10.1016/j.jece.2019.103313 [2] Chen Donghui. Nnual evaluation for vanadium industry in 2018[J]. Hebei Metallurgy, 2019,(8):5−15. (陈东辉. 钒产业2018年年度评价[J]. 河北冶金, 2019,(8):5−15. [3] Cui X, Zhang G, Chen X, et al. Purification of V2O5 and its application in all-vanadium redox flow batteries[J]. Materials Research Express, 2019,6(8):085552. doi: 10.1088/2053-1591/ab27e4 [4] Li H, Tian H, Chang T H, et al. High-purity V2O5 nanosheets synthesized from gasification waste: flexible energy storage devices and environmental assessment[J]. ACS Sustainable Chemistry & Engineering, 2019,7(14):12474−12484. [5] Choi C, Kim S, Kim R, et al. A review of vanadium electrolytes for vanadium redox flow batteries[J]. Renewable and Sustainable Energy Reviews, 2017,69:263−274. doi: 10.1016/j.rser.2016.11.188 [6] He D, Feng Q, Zhang G, et al. An environmentally-friendly technology of vanadium extraction from stone coal[J]. Minerals Engineering, 2007,20(12):1184−1186. doi: 10.1016/j.mineng.2007.04.017 [7] Wang Bin. Study on extraction of vanadium from acid leaching solution of stone coal with ion exchange resin[J]. Iron Steel Vanadium Titanium, 2007,28(1):22−25. (王斌. 石煤浸出液离子交换法提钒的研究[J]. 钢铁钒钛, 2007,28(1):22−25. doi: 10.3969/j.issn.1004-7638.2007.01.005 [8] Thomas J, Surender G D, Reddy M L P. Solvent extraction separation of vanadium(V) from multimetal chloride solutions using tributyl phosphate[J]. Separation Science & Technology, 2003,38(15):3761−3774. [9] Zhao J, Hu Q, Li Y, et al. Efficient separation of vanadium from chromium by a novel ionic liquid-based synergistic extraction strategy[J]. Chemical Engineering Journal, 2015,264:487−496. doi: 10.1016/j.cej.2014.11.071 [10] Gaballah I, Djona M. Recovery of Co, Ni, Mo, and V from unroasted spent hydrorefining catalysts by selective chlorination[J]. Metallurgical and Materials Transactions B, 1995,26(1):41−50. doi: 10.1007/BF02648975 [11] McCarley R E, Roddy J W. The preparation of high purity vanadium pentoxide by a chlorination procedure[J]. Journal of the Less Common Metals, 1960,2(1):29−35. doi: 10.1016/0022-5088(60)90036-9 [12] Jiang D, Zhang H, Xu H, et al. Chlorination and purification of vanadium pentoxide with anhydrous aluminum chloride[J]. Journal of Alloys and Compounds, 2017,709:505−510. doi: 10.1016/j.jallcom.2017.03.123 [13] Brocchi E A, Navarro R C S, Moura F J. A chemical thermodynamics review applied to V2O5 chlorination[J]. Thermochimica Acta, 2013,559:1−16. doi: 10.1016/j.tca.2013.01.025 [14] Gaballah I, Djona M, Allain E. Kinetics of chlorination and carbochlorination of vanadium pentoxide[J]. Metallurgical & Materials Transactions B, 1995,26(4):711−718. [15] Du G, Fan C, Yang H, et al. Selective extraction of vanadium from pre-oxidized vanadium slag by carbochlorination in fluidized bed reactor[J]. Journal of Cleaner Production, 2019,237:117765. doi: 10.1016/j.jclepro.2019.117765 [16] Mink G, Bertóti I, Székely T. Chlorination of V2O5 by CCl4 Adsorption and steady state reaction[J]. Reaction Kinetics and Catalysis Letters, 1985,27(1):33−38. doi: 10.1007/BF02064456 [17] Zheng H, Sun Y, Lu J, et al. Vanadium extraction from vanadium-bearing titanomagnetite by selective chlorination using chloride wastes (FeCl x)[J]. Journal of Central South University, 2017,24(2):311−317. doi: 10.1007/s11771-017-3432-x [18] Zhang Y, Hu Y, Bao S. Vanadium emission during roasting of vanadium-bearing stone coal in chlorine[J]. Minerals Engineering, 2012,30:95−98. doi: 10.1016/j.mineng.2012.02.003 [19] (莫畏, 邓国珠, 罗方承. 钛冶金(第二版)[M]. 北京: 冶金工业出版社, 2007.)Mo Wei, Deng Guozhu, Luo Fangcheng. Titanium metallurgy[M]. Beijing: Metallurgical Industry Press, 2007. [20] Lynch D C. Conversion of VOCl3 to VOCl2 in liquid TiCl4[J]. Metallurgical and Materials Transactions B, 2002,33(1):142−146. doi: 10.1007/s11663-002-0096-0 [21] Uda T, Okabe T H, Waseda Y, et al. Contactless electrochemical reduction of titanium (II) chloride by aluminum[J]. Metallurgical and Materials Transactions B, 2000,31(4):713−721. doi: 10.1007/s11663-000-0110-3 [22] Liang Qiang. The engineering design issues of removing vanadium from raw titanium tetrachloride by copper wire[J]. Guizhou Science, 2011,29(4):65−68. (梁强. 四氯化钛精制中铜丝除钒工程设计问题的探讨[J]. 贵州科学, 2011,29(4):65−68. doi: 10.3969/j.issn.1003-6563.2011.04.014 [23] Zhou Li. Study on the organics pretreatment of crude titanium tetrachloride with high content of vanadium for vanadium removal[J]. Iron Steel Vanadium Titanium, 2017,38,(4):24−28. (周丽. 高含钒粗四氯化钛有机物预处理除钒工艺研究[J]. 钢铁钒钛, 2017,38,(4):24−28. doi: 10.7513/j.issn.1004-7638.2017.04.005 [24] Long Xiang, Li Haiyan, Yang Zheng, et al. Experimental study on removing vanadium from titanium tetrachloride with mineral oil[J]. Hydrometallurgy of China, 2018,37(4):59−62. (龙翔, 李海艳, 杨振, 等. 用矿物油从四氯化钛中去除钒试验研究[J]. 湿法冶金, 2018,37(4):59−62. [25] Yang Yibang, Wang Fuwen, Li Baojin, et al. Development and evaluation of process for removing vanadium from crude titanium tetrachloride[J]. Materials Reports, 2012,26(1):157−160. (杨易邦, 王富文, 李保金, 等. 粗四氯化钛除钒工艺进展及评价[J]. 材料导报, 2012,26(1):157−160. [26] Fan C, Yang H, Zhu Q, et al. Selective hydrolysis of trace TiCl4 from VOCl3 for preparation of high purity V2O5[J]. Separation and Purification Technology, 2017,185:196−201. doi: 10.1016/j.seppur.2017.05.002 [27] Lassègue P, Noé L, Monthioux M, et al. Fluidized bed chemical vapor deposition of copper nanoparticles on multi-walled carbon nanotubes[J]. Surface and Coatings Technology, 2017,331:129−136. doi: 10.1016/j.surfcoat.2017.10.046 [28] Ke Chen Z, Jing Wang L, Wang H, et al. Effect of microstructure on impact resistance of chemical vapor deposited SiC coating on graphite substrate[J]. Surface and Coatings Technology, 2019,380:125076. doi: 10.1016/j.surfcoat.2019.125076 [29] Liu X, Wang W, Zhang H, et al. La-doped diamond films prepared through microwave plasma chemical vapor deposition[J]. Thin Solid Films, 2019,692:137620. doi: 10.1016/j.tsf.2019.137620 [30] Vahlas C, Caussat B, Serp P, et al. Principles and applications of CVD powder technology[J]. Materials Science and Engineering: R: Reports, 2006,53(1−2):1−72. doi: 10.1016/j.mser.2006.05.001 [31] Gao J M, Song X F, Hu J, et al. Superhydrophobic graphenic carbon nanowalls fabricated by one-step PECVD[J]. Materials Letters, 2016,184:273−277. doi: 10.1016/j.matlet.2016.07.127 [32] Fan C, Xu J, Yang H, et al. High-purity, low-Cl V2O5 via the gaseous hydrolysis of VOCl3 in a fluidized bed[J]. Particuology, 2020,49:9−15. doi: 10.1016/j.partic.2018.12.005 [33] (范川林, 朱庆山, 杨海涛. 一种氯化法制备高纯五氧化二钒粉体的系统及方法, 中国: CN107555478 A[P]. 2017-06-13.)Fan Chuanlin, Zhu Qingshan, Yang Haitao. A system and method for preparation of high purity vanadium pentoxide powder by chlorination method, China: CN107555478 A[P]. 2017-06-13. -





 下载:
下载: