Preparation of fibrous titanium-lithium ion sieve precursor with skin collagen as the template
-
摘要: 为解决传统的粉末状钛锂离子筛渗透性和流动性差的问题,以皮胶原作为模板剂,廉价易得的Ti(SO4)2为钛源,乙酸锂为锂源,经皮胶原负载钛、锂及固相反应制备具有纤维结构的钛锂离子筛前驱体(Li2TiO3)。探究加入不同试剂、不同处理方法、不同pH值等因素对合成钛锂离子筛前驱体(Li2TiO3)的影响。采用扫描电子显微镜和X射线衍射仪对试样进行了表征。结果表明:加入单宁,控制试验pH=4.0在750 ℃条件下保温8 h制备的钛锂离子筛前驱体纤维结构较好,晶相组成为单斜结构β-Li2TiO3。Abstract: In order to solve the poor permeability and fluidity of traditional powder titanium-lithium ion sieve, fibrous precursor of titanium-lithium ion sieve (Li2TiO3) was prepared via the solid-state reaction with skin collagen as the template, Ti(SO4)2 and lithium acetate as the titanium and lithium source respectively. The effects of reagent, treatment method and pH value on the synthesis of titanium-lithium ion sieve precursor were investigated. The samples were characterized by scanning electron microscopy (SEM) and X-ray diffraction (XRD). The results show that a good fibrous precursor of titanium-lithium ion sieve can be obtained by adding tannin, controlling pH=4.0 and holding at 750 ℃ for 8 h, with the obtained sample composed of monoclinic β-Li2TiO3.
-
Key words:
- titanium-lithium ion sieve /
- precursor /
- collagen /
- fiber structure /
- tannin
-
0. 引言
锂产品被广泛应用于锂电池、化学工业、新能源、合金制作等不同领域[1-2]。有报道称,锂是极具前景的战略资源[3]。随着锂资源需求量的逐年增加,固态锂富矿资源远远不能满足市场需求。从液态锂资源中提锂成本更低[4],其中以卤水生产锂盐比用矿石生产锂盐综合成本节省30%~50%[5]。中国是盐湖锂资源大国,蕴藏着丰富的液态锂矿,其中盐湖锂储量约占全国锂总储量的78%,极具开发和利用价值。然而,中国的盐湖锂资源镁含量高,锂含量较低,这给我国盐湖锂资源的开发造成了极大困难[6]。同样,全球蕴藏了丰富的锂资源,但是只有很少的锂资源得以开发利用[3]。
据文献[7-12]报道,目前从盐湖提锂的方法多种多样,主要包括:沉淀法、溶剂萃取法、膜分离法、电渗析法、纳滤法、离子筛吸附法等。离子筛吸附法是目前从高镁锂盐湖中提取低品位锂的研究热点,该法又分为有机和无机两类,目前更受研究者青睐的是无机吸附法,其提取过程大致可以分为前驱体的制备和锂离子的分离。在提取过程中首先要制备含有锂离子的前驱体化合物,然后再用洗脱剂将锂离子抽离出来。最后在不改变晶型的情况下得到与含有锂离子相匹配的空隙化合物,所得材料对于锂离子有较强记忆效应[13-14],因此该材料对锂离子表现出高度选择性。
目前锂离子筛吸附法提锂研究得最多且应用前景较好的主要为锰系离子筛和钛系离子筛[15-18]。锰系离子筛存在溶损率高和歧化效应等问题,钛系锂离子筛与之相比,更具有优势。钛系锂离子筛主要有两类。一类[19]具有尖晶石结构,如H4Ti5O12,由于钛的化合价为正四价,在吸附过程中不会存在因钛的变价而导致钛溶损的现象,所以该类钛系锂离子在吸附过程中比较稳定。另一类[20]是以H2TiO3为主的具有层状结构的钛系锂离子筛。但是据文献[21]报道,在制备前驱体时很难获得纯的尖晶石型 Li4Ti5O12,产物中经常混有金红石型TiO2和Li2TiO3,并且Li4Ti5O12中锂含量不如Li2TiO3高(尖晶石型钛锂离子筛的理论交换吸附容量不如偏钛酸型高)。因此,Li2TiO3更加适合作为钛锂离子筛前驱体[22-23]。但目前制备的钛系锂离子筛大多为粉末状,其存在渗透性和流动性差的问题,难以实现工业化应用。
锂离子筛的形状对性能有重要的影响[24]。纤维状或管状的锂离子筛,具有良好的几何柔韧性,并且纤维状锂离子筛克服了粉末状锂离子筛渗透性和流动性差的问题,更有利于实现工业化应用。因此,构建纤维结构的钛锂离子筛具有十分重要的意义。胶原纤维具有特殊的分子结构和空间结构,能与 Al(Ⅲ)、Fe(Ⅲ)、Cr(Ⅲ)、Zr(Ⅳ)、Ti(Ⅳ)等金属形成稳定的配位化合物[25]。因此以胶原纤维为模板,能够制备具有特定形貌的材料[26]。据我们了解,以胶原纤维为模板制备具有纤维结构的偏钛酸型钛锂离子筛还未见报道。笔者采用天然生物质材料皮胶原为模板,廉价易得的硫酸钛为钛鞣剂,乙酸锂为锂源,采用模板法合成出具有纤维结构的钛锂离子筛前驱体。
1. 试验材料与方法
1.1 试验材料与设备
表 1 主要试验试剂Table 1. The main reagents化学试剂 规格 生产厂家规格 皮胶原 分析纯(AR) 中国林业科学研究院林产化学工业研究所科技开发总公司单宁化工实验室 柠檬酸三钠 分析纯(AR) 成都市科龙化工试剂厂 柠檬酸(一水) 分析纯(AR) 成都市科龙化工试剂厂 氯化钠 分析纯(AR) 成都金山化学试剂有限公司 甲酸 分析纯(AR) 成都金山化学试剂有限公司 无水乙醇 分析纯(AR) 成都金山化学试剂有限公司 硫酸钛 分析纯(AR) 国药集团化学试剂有限公司 碳酸氢钠 分析纯(AR) 成都市科龙化工试剂厂 乙酸锂 分析纯(AR) 山东西亚化学工业有限公司 硫酸 分析纯(AR) 成都金山化学试剂有限公司 三乙醇胺 分析纯(AR) 成都金山化学试剂有限公司 单宁 分析纯(AR) 成都市新都区木兰镇工业开发区 戊二醛25% 分析纯(AR) 成都市科隆花心品有限公司 表 2 主要试验仪器Table 2. The main experimental instrument试验仪器 型号 生产厂家 扫描电子显微镜 VEGA3SBH TESCAN 磁力搅拌器 HJ-4 常州未来仪器制造有限公司 pH计 pH-902 常州爱德克斯仪器仪表有限公司 ZNCL-GS智能磁力搅拌器 ZNCL-GS240*150 上海予申仪器有限公司 电热鼓风干燥箱 101-2BS 天津宏诺仪器有限公司 优普系列
超纯水器UPH-IV-10T 成都超纯科技有限公司 精密节能电炉 SX2-5-12TP 济南精密科学仪器仪表有限公司 低速大容量
离心机TDL-5-A 上海安亭科学仪器厂 超声波清洗机 QT系列 天津市瑞普电子仪器公司 电子天平 JA5003 上海舜宇恒平科学仪器有限公司 X射线衍射仪 DX-2700 丹东浩圆仪器有限公司 1.2 试验方法
1.2.1 缓冲溶液的配制
分别称取10.5 g的柠檬酸(一水)和0.025 g的柠檬酸三钠,一同放入50 mL的小烧杯中溶解,然后转移至500 mL容量瓶,多次冲洗小烧杯并将冲洗液通过玻璃棒引流转移至容量瓶摇匀后即可得到柠檬酸-柠檬酸三钠缓冲溶液。
1.2.2 钛鞣剂的制备
用电子天平称取2.4 g的硫酸钛轻轻倒入250 mL的烧杯中,加入30 mL左右上述柠檬酸-柠檬酸三钠缓冲溶液,随后再用饱和碳酸氢钠溶液将溶液pH值调为2.0左右,即得到钛鞣剂。
1.2.3 前驱体的制备
钛系锂离子筛前驱体(Li2TiO3)的制备通过在皮胶原负载钛的过程中加入锂源,然后采用固相法直接合成前驱体。
1) 称取1.5 g的皮胶原和0.6 g氯化钠于250 mL烧杯中,加入30 mL蒸馏水,用质量分数为25%的稀硫酸和甲酸按质量浓度比3∶1配制而成的混酸将溶液的pH值调为2.0左右,在常温下搅拌4 h。
2) 加入制得的钛鞣剂,在常温下搅拌8 h。
接下来,分别采用以下四种方法得到钛系锂离子筛前驱体,探究不同方法下皮胶原为模板制备纤维状钛锂离子筛前驱体的形貌结构,四种方法中相关参数的确定是依据前期大量试验的结果。四种探究方法如下:
方法一:用饱和碳酸氢钠溶液将pH值调为4左右,在38 ℃的水浴条件下搅拌2 h。用低速大容量离心机在4000 r/min的条件下离心3 min,先用蒸馏水洗涤两次,再用乙醇洗涤两次,然后在乙醇中浸泡4 h,再加入乙酸锂,然后补加一定量的无水乙醇继续在常温下搅拌4 h,最后在50 ℃的条件下干燥。将干燥后加入锂源的皮胶原负载钛放在马弗炉中煅烧,温度为750 ℃,保温时间为8 h,升温速度为5 ℃/min。
方法二:加入乙酸锂在无水乙醇中搅拌4 h,超声处理20 min,其余部分与方法一相同。
方法三:加入乙酸锂搅拌4 h后加入不同试剂(如:单宁,戊二醛,三乙醇胺),其余部分与方法一相同。
方法四:加入钛鞣剂后控制不同pH值,分别为pH=3.8、pH=4.0、pH=4.2。
2. 结果与讨论
2.1 皮胶原形貌
皮胶原通常是将具有天然纤维状蛋白质的牛皮经过纯化、磨碎等加工制得的白色绒状物质,本研究中皮胶原购于中国林业科学研究院林产化学工业研究所科技开发总公司单宁化学研究室,皮胶原是用牛皮制备的,其具有良好的纤维结构[27],如图1所示。可以看出,皮胶原是由几条链形成的螺旋状结构组成,呈现出明显的纤维结构。所以以其为模板有望制备纤维结构的功能性材料。
2.2 皮胶原负载钛和锂源制备前驱体
2.2.1 不同处理方法
图2显示了在锂钛比为2∶1时,加入乙酸锂后采用不同方法进行的处理,然后再在750 ℃下煅烧8 h制得的钛锂离子筛前驱体的SEM图像。图2a是直接制备前驱体,不做任何处理的空白组;图2b为在无水乙醇中搅拌4 h、超声波处理20 min后烘干产品的SEM图像;通过分析,可以发现处理方法不同,得到的产品形貌不同。加入锂源时采用超声波处理20 min再烘干制备的钛锂离子筛前驱体样品中纤维数量较少且纤维结构有断裂现象,并没有达到预期的现象,说明超声波处理可能破坏产品的纤维结构。在进行超声处理时,液体间的超强冲击波随之产生,在这样的情况下液体分子表面会在小范围内瞬时产生大于5 000 K的高温和大于50 MPa高压,因此强烈的冲击波可能会破坏产品的纤维结构。文献[28]表明,进行超声处理时,超声时间和超声频率对产物结构均有不同的影响,此外超声分散的效果可能与反应物本身性质有关。
2.2.2 不同试剂处理
图3是在锂钛比为2∶1、乙酸锂为锂源、并分别采用单宁、戊二醛、三乙醇胺处理并在750 ℃温度下煅烧8 h制得的钛锂离子筛前驱体(Li2TiO3)的SEM图像。单宁在与蛋白质和金属离子反应方面表现出较高的化学反应活性,这为通过单宁的加入将皮胶原纤维改性提供了可能,也为提高产品吸附性能得到纯度较高的产品提供了科学理论[29]。研究[30]表明,单宁与胶原纤维结合过程中单宁分别借助疏水键和多点氢键达到与皮胶原纤维的有机结合。由于金属离子缺乏有效与皮胶原纤维结合的邻位酚羟基结构,往往不能与胶原纤维形成多点氢键结合,因此胶原纤维与金属离子结合效果不佳,尤其是在本实验中胶原纤维与锂离子难以有效结合导致最终产品吸附量比较低。但单宁具有特殊的化学结构,这使它既能与多种金属离子发生络合反应,又能与皮胶原纤维有效结合,因此有可能利用胶原纤维和单宁研究制备更好的钛锂离子筛前驱体。
胶原纤维主要成分是蛋白质,而蛋白质很容易遭到微生物和化学物质的侵蚀,且胶原纤维热稳定性差,因此为了达到理想实验效果,很有必要对胶原纤维进行适当的化学改性。本实验拟采用采用25%戊二醛对胶原纤维进行化学改性以增强胶原纤维的耐热性和耐腐蚀性,以保护所得钛锂离子筛前驱体的纤维结构。
三乙醇胺是一种非离子表面活性剂,在很多领域都有所应用,通常可用做良好的分散剂、润滑剂、固化剂等,其本身含有三个羟基并拥有较强的极性,能够有效降低液-固界面的自由能。
图3a是没有加入任何额外试剂时的SEM图像,图3b、3c、3d是样品在用乙醇做分散剂浸泡4 h时加入0.5 mL25%戊二醛、0.5 g单宁、1 mL三乙醇胺所得产品SEM图像。通过图像分析可知:3a中钛锂离子筛前驱体产品呈长条絮状,产品表面光滑,总体而言具有一定的纤维结构。3b中所得产品纤维结构严重遭到破坏,且表面有小颗粒物质存在,产品纤维结构较3a差得多,可能原因是戊二醛的浓度较高、用量较大。3c中产品纤维结构为长条状,纤维数量较多,纤维间距较小,对比可以发现加入单宁后仍然具有较好的纤维结构。对比3a和3d可以发现,3d中产品纤维几乎呈大颗粒状,几乎没有纤维结构,造成该现象的原因可能有:一是在该条件下煅烧温度较高,有机碳会燃烧导致产品纤维结构大面积遭到破坏,并使得纤维结构被破坏成小颗粒状或者烧结形成大块的颗粒。二是三乙醇胺的用量较多,关于三乙醇胺用量对产品的影响,相关文献中有类似报道[31],也就是三乙醇胺用量较多时会使得皮胶原纤维的硬度、拉升强度、断裂伸长率等降低从而导致力学性能降低。对比3a、3b、3c和3d可得,在锂钛比为2∶1、乙酸锂为锂源、用单宁处理并在750 ℃温度下煅烧8 h制得的产品具有良好的纤维结构。
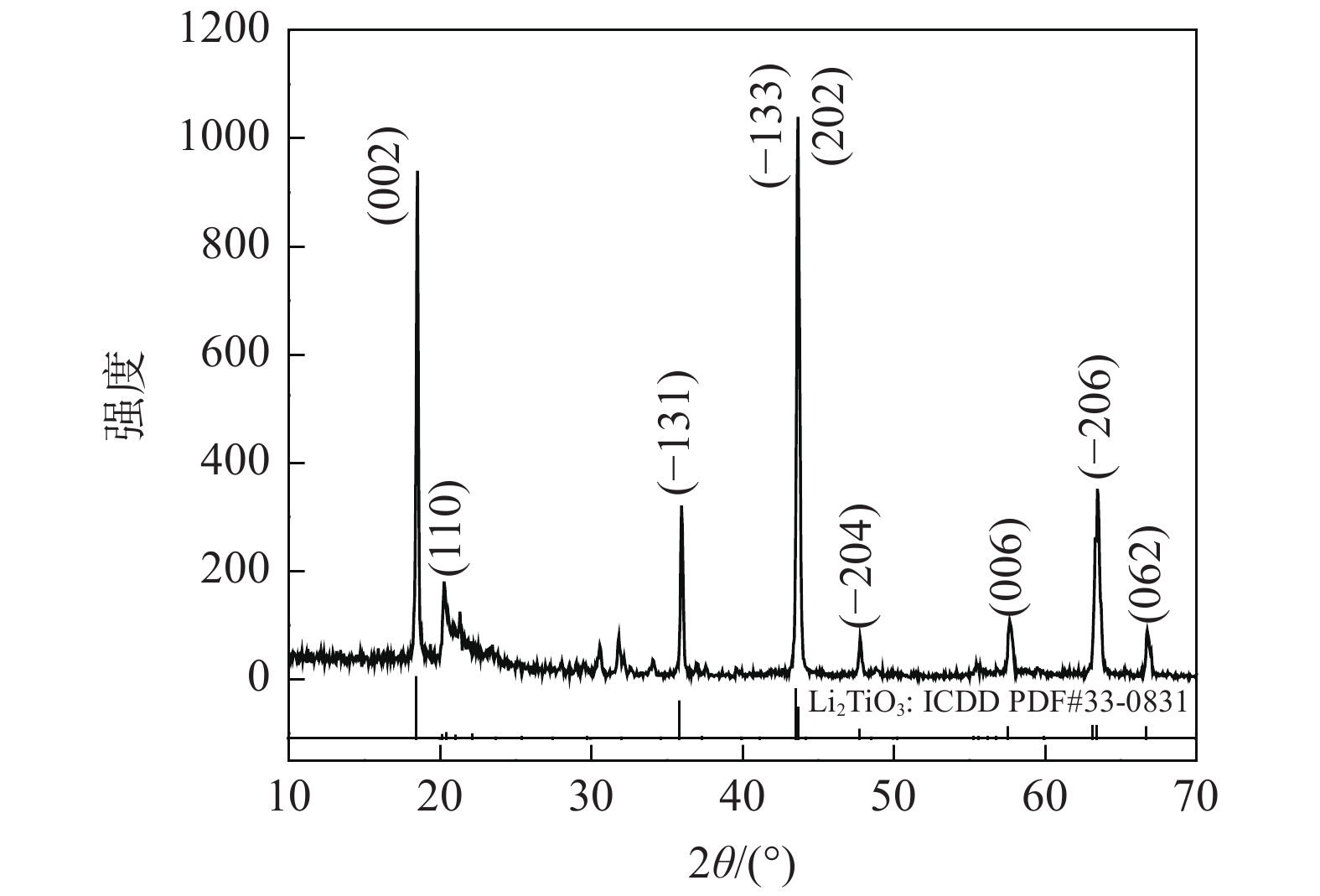
图4显示了在锂钛比为2∶1时,采用第二类方法中方法三,以乙酸锂为锂源,加入单宁在750 ℃下煅烧8 h制得的钛锂离子筛前驱体(Li2TiO3)的XRD图谱,样品的XRD图谱的出峰位置与β-Li2TiO3的标准卡片(Li2TiO3: ICDD PDF #33-0381)的出峰位置基本相同,且(002)、(110)、(−133)、(−204)、(202)、(−206)等晶面衍射峰都有出现。2θ位于43.5o左右的衍射峰强度超过了2θ位于18.5o左右的衍射峰强度,而β-Li2TiO3的标准卡片(Li2TiO3: ICDD PDF #33-0381)中2θ=18.5o处的衍射峰为最强衍射峰。这是因为(−133)和(202)这两个晶面衍射峰位置极相近,衍射峰在此处叠加在一起的缘故。总体来说,图4中衍射峰基本上都是β-Li2TiO3的衍射峰,由此可以知道该样品主要晶相成分为单斜结构β-Li2TiO3,这从另一个角度证实了本次试验的可行性。
2.2.3 不同的pH处理
图5显示了在锂钛比为2∶1、乙酸锂为锂源、在加入钛鞣剂对反应体系进行不同的pH处理并在750 ℃温度下煅烧8 h制得的钛锂离子筛前驱体(Li2TiO3)的SEM图像。图5a是将pH控制在3.8时所得前驱体的扫描电镜图片,图5b为pH=4.2所得前驱体扫描电镜图片,图5c为pH=4.0所得前驱体扫描电镜图片。可以看出,图5a中前驱体基本保留了纤维结构轮廓,但是纤维间距变小,产品表面光滑且有少量颗粒物质存在。而图5b中很难看出其纤维结构,破坏更为严重,产品呈块状。图5c中纤维数量较多,纤维间空隙较小。综合分析可知,试验时将pH控制在pH=4.0时所得产品纤维结构最好,同等情况下pH一定范围内偏小的结果要好于偏大的。
3. 结论
1)以皮胶原作为模板剂,硫酸钛为钛源,乙酸锂为锂源,采用不同处理方法(超声处理20 min),所得钛锂离子筛前驱体纤维结构形貌不理想。
2)加入单宁,控制试验pH=4.0在750 ℃条件下保温8 h制备的产物纤维结构较好,物相组成为单斜结构β-Li2TiO3。
3)纤维状钛锂离子筛前驱体能够增强其渗透性和流动性,对其实现离子交换柱操作具有重要意义,下一步工作中,我们将研究纤维状钛锂离子筛前驱体的洗脱转型、交换吸附性能,以及采用离子交换柱研究其动态交换吸附、洗脱再生、循环使用性能,为其应用奠定坚实的基础。
-
表 1 主要试验试剂
Table 1. The main reagents
化学试剂 规格 生产厂家规格 皮胶原 分析纯(AR) 中国林业科学研究院林产化学工业研究所科技开发总公司单宁化工实验室 柠檬酸三钠 分析纯(AR) 成都市科龙化工试剂厂 柠檬酸(一水) 分析纯(AR) 成都市科龙化工试剂厂 氯化钠 分析纯(AR) 成都金山化学试剂有限公司 甲酸 分析纯(AR) 成都金山化学试剂有限公司 无水乙醇 分析纯(AR) 成都金山化学试剂有限公司 硫酸钛 分析纯(AR) 国药集团化学试剂有限公司 碳酸氢钠 分析纯(AR) 成都市科龙化工试剂厂 乙酸锂 分析纯(AR) 山东西亚化学工业有限公司 硫酸 分析纯(AR) 成都金山化学试剂有限公司 三乙醇胺 分析纯(AR) 成都金山化学试剂有限公司 单宁 分析纯(AR) 成都市新都区木兰镇工业开发区 戊二醛25% 分析纯(AR) 成都市科隆花心品有限公司 表 2 主要试验仪器
Table 2. The main experimental instrument
试验仪器 型号 生产厂家 扫描电子显微镜 VEGA3SBH TESCAN 磁力搅拌器 HJ-4 常州未来仪器制造有限公司 pH计 pH-902 常州爱德克斯仪器仪表有限公司 ZNCL-GS智能磁力搅拌器 ZNCL-GS240*150 上海予申仪器有限公司 电热鼓风干燥箱 101-2BS 天津宏诺仪器有限公司 优普系列
超纯水器UPH-IV-10T 成都超纯科技有限公司 精密节能电炉 SX2-5-12TP 济南精密科学仪器仪表有限公司 低速大容量
离心机TDL-5-A 上海安亭科学仪器厂 超声波清洗机 QT系列 天津市瑞普电子仪器公司 电子天平 JA5003 上海舜宇恒平科学仪器有限公司 X射线衍射仪 DX-2700 丹东浩圆仪器有限公司 -
[1] Xu X, Chen Y, Wan P, et al. Extraction of lithium with functionalized lithium ion-sieves[J]. Progress in Materials Science, 2016,(84):276−313. [2] Gruber P W, Medina P A, Keoleian G A, et al. Global lithium availability, a constraint for electric vehicles[J]. Journal of Industrial Ecology, 2011,15(5):760−775. doi: 10.1111/j.1530-9290.2011.00359.x [3] Grosjean C, MirandaI P H, Perrin M, et al. Assessment of world lithium resources and consequences of their geographic distribution on the expected development of the electric vehicle industry[J]. Renewable and Sustainable Energy Reviews, 2012,16(3):1735−1744. doi: 10.1016/j.rser.2011.11.023 [4] Ogawa Y, Koibuchi H, Suto K, et al. Effects of the chemical compositions of salars de Uyuni and Atacama brines on lithium concentration during evaporation[J]. Resource Geology, 2014,4(2):91−101. [5] Meshram P, Pandey B D, Mankhand T R. Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: a comprehensive review[J]. Hydrometallurgy, 2014,150:192−208. doi: 10.1016/j.hydromet.2014.10.012 [6] Liu Yuanhui, Deng Tianlong. Progresses on the process and technique of lithium recovery from salt lake brines around the world[J]. World Sci-Tech R & D, 2006,28(5):69−75. (刘元会, 邓天龙. 国内外从盐湖卤水中提锂工艺技术研究进展[J]. 世界科技研究与发展, 2006,28(5):69−75. doi: 10.3969/j.issn.1006-6055.2006.05.010 [7] Lemaire J, Svecova L, Lagallarde E, et al. Lithium recovery from aqueous solution by sorption/desorption[J]. Hydrometallurgy, 2014,143:1−11. doi: 10.1016/j.hydromet.2013.11.006 [8] Wu X, Wen Z, Lin B, et al. Sol-gel synthesis and sintering of nano-size Li2TiO3 powder[J]. Materials Letters, 2008,62(6−7):837−839. doi: 10.1016/j.matlet.2007.06.073 [9] Chitrakar R, Makita Y, Ooi K, et al. Synthesis of iron-doped manganese oxides with an ion-sieve property: lithium adsorption from bolivian brine[J]. Industrial & Engineering Chemistry Research, 2014,53(9):3682−3688. [10] Sun S Y, Cai L J, Nie X Y, et al. Separation of magnesium and lithium from brine using a desal nanofiltration membrane[J]. Journal of Water Process Engineering, 2015,7:210−217. doi: 10.1016/j.jwpe.2015.06.012 [11] Song J, Li X M, Zhang Y, et al. Hydrophilic nanoporous ion-exchange membranes as a stabilizing barrier for liquid-liquid membrane extraction of lithium ions[J]. Journal of Membrane Science, 2014,471:372−380. doi: 10.1016/j.memsci.2014.08.010 [12] Ji Z Y, Chen Q B, Yuan J S, et al. Preliminary study on recovering lithium from high Mg2+/Li+ ratio brines by electrodialysis[J]. Separation and Purification Technology, 2017,172:168−177. doi: 10.1016/j.seppur.2016.08.006 [13] Wang L, Meng C G, Ma W. Study on Li+ uptake by lithium ion-sieve via the pH techniqu[J]. Colloids & Surfaces A-Physicochemical & Engineering Aspects, 2009,334(1−3):34−39. [14] Zhang Q H, Li S, Sun S Y, et al. Lithium selective adsorption on 1-D MnO nanostructure ion-sieve[J]. Advanced Powder Technology, 2009,20(5):432−437. doi: 10.1016/j.apt.2009.02.008 [15] Bai C, Guo M, Zhang H F, et al. The research progress of extracting lithium from brine by lithium ion sieve[J]. Chemical Industry and Engineering Progress, 2017,36(3):802−809. [16] Shi Xichang, Yu Liangliang, Chen Baizhen, et al. Preparation and adsorption property of spinel-type lithium ion-sieve[J]. Journal of Central South University (Science and Technology), 2011,42(8):2198−2203. (石西昌, 余亮良, 陈白珍, 等. 尖晶石型锂离子筛的制备及其吸附性能[J]. 中南大学学报(自然科学版), 2011,42(8):2198−2203. [17] Chitrakar R, Kanoh H, Miyai Y, et al. Recovery of lithium from seawater using manganese oxide adsorbent (H1.6Mn1.6O4) derived from Li1.6Mn1.6O4[J]. Industrial & Engineering Chemistry Research, 2001,40(9):2054−2058. [18] Zhang L Y, Zhou D L, He G, et al. Synthesis of H2TiO3-lithium adsorbent loaded on ceramic foams[J]. Materials Letters, 2015,145:351−354. doi: 10.1016/j.matlet.2015.01.142 [19] Park K, Benayad A, Kang D, et al. Nitridation-driven conductive Li4Ti5O12 for lithium ion batteries[J]. Journal of the American Chemical Society, 2008,130(45):14930−14931. doi: 10.1021/ja806104n [20] Xu X, Zhou Y, Fan M, et al. Lithium adsorption performance of a three-dimensional porous H2TiO3-type lithium ion-sieve in strong alkaline Bayer liquor[J]. RSC Advances, 2017,7:18883−18891. doi: 10.1039/C7RA01056G [21] Nugroho A, Kim S J, Chung K Y, et al. Facile synthesis of nanosized Li4Ti5O12 in supercritical water[J]. Electrochemistry Communications, 2011,13(6):650−653. doi: 10.1016/j.elecom.2011.03.037 [22] Onodera Y, Iwasaki T, Hayashi H, et al. A new inorganic material with high selective adsorbability for Li+[J]. Journal of the Ceramic Society of Japan, 1989,97(1129):888−894. doi: 10.2109/jcersj.97.888 [23] Zhang L F, Chen B Z, Shi X C, et al. Synthesis and adsorption property of H2TiO3 type adsorbent[J]. The Chinese Journal of Nonferrous Metals, 2010,20(9):1850−1854. [24] Chung W J, Torrejos R E C, Park M J, et al. Continuous lithium mining from aqueous resources by an adsorbent filter with a 3D polymeric nanofiber network infused with ion sieves[J]. Chemical Engineering Journal, 2017,309:49−62. doi: 10.1016/j.cej.2016.09.133 [25] Deng D H, Wu H, Liao X P, et al. Synthesis of unique mesoporous ZrO2-carbon fiber from collagen fiber[J]. Microporous and Mesoporous Materials, 2008,116(1−3):705−709. doi: 10.1016/j.micromeso.2008.05.018 [26] Cai Li. Synthesis and photo-catalytic activity of mesoporous TiO2 fiber using collagen fiber as a template[J]. Journal of Functional Materials, 2013,44(23):3447−3451. (蔡莉. 胶原纤维为模板制备介孔TiO2纤维及光催化活性研究[J]. 功能材料, 2013,44(23):3447−3451. doi: 10.3969/j.issn.1001-9731.2013.23.020 [27] Zhang Liyuan, You Yaohui, You Jia, et al. Preparation and photocatalytic performance of fibrous Gd-doped TiO2 using collagen fiber as template[J]. Journal of Synthetic Crystals, 2019,48(2):312−320. (张理元, 由耀辉, 尤佳, 等. 胶原纤维为模板制备Gd掺杂纤维状二氧化钛及光催化性能研究[J]. 人工晶体学报, 2019,48(2):312−320. doi: 10.3969/j.issn.1000-985X.2019.02.024 [28] Zhang Bin, Chen Tijun, Wang Lingyun, et al. Study on ultrasonic dispersion of graphene nanoplatelets[J]. Journal of Functional Materials, 2019,50(8):8133−8139. (张斌, 陈体军, 王凌云, 等. 石墨烯纳米片超声分散的研究[J]. 功能材料, 2019,50(8):8133−8139. doi: 10.3969/j.issn.1001-9731.2019.08.019 [29] Schofield P, Mbugua D M, Pell A N. Analysis of condensed tannins: a review[J]. Animal Feed Science and Technology, 2001,91(1−2):21−40. doi: 10.1016/S0377-8401(01)00228-0 [30] Shi B, He X Q, Haslam E. Pofyphenol-Gelatin interaction[J]. Journal of American Leather Chemists Association, 1994,89(4):98−104. [31] Tao Yuhu. Preparation and properties of triethanolamine modified basalt fiber/natural rubber composites[J]. Science & Technology Vision, 2018,(10):256−258. (陶玉虎. 三乙醇胺改性玄武岩纤维/天然橡胶复合材料的制备及性能研究[J]. 科技视界, 2018,(10):256−258. -





 下载:
下载:






 下载:
下载: