Production cost of current titanium metallurgical process and possibility of new alternative process
-
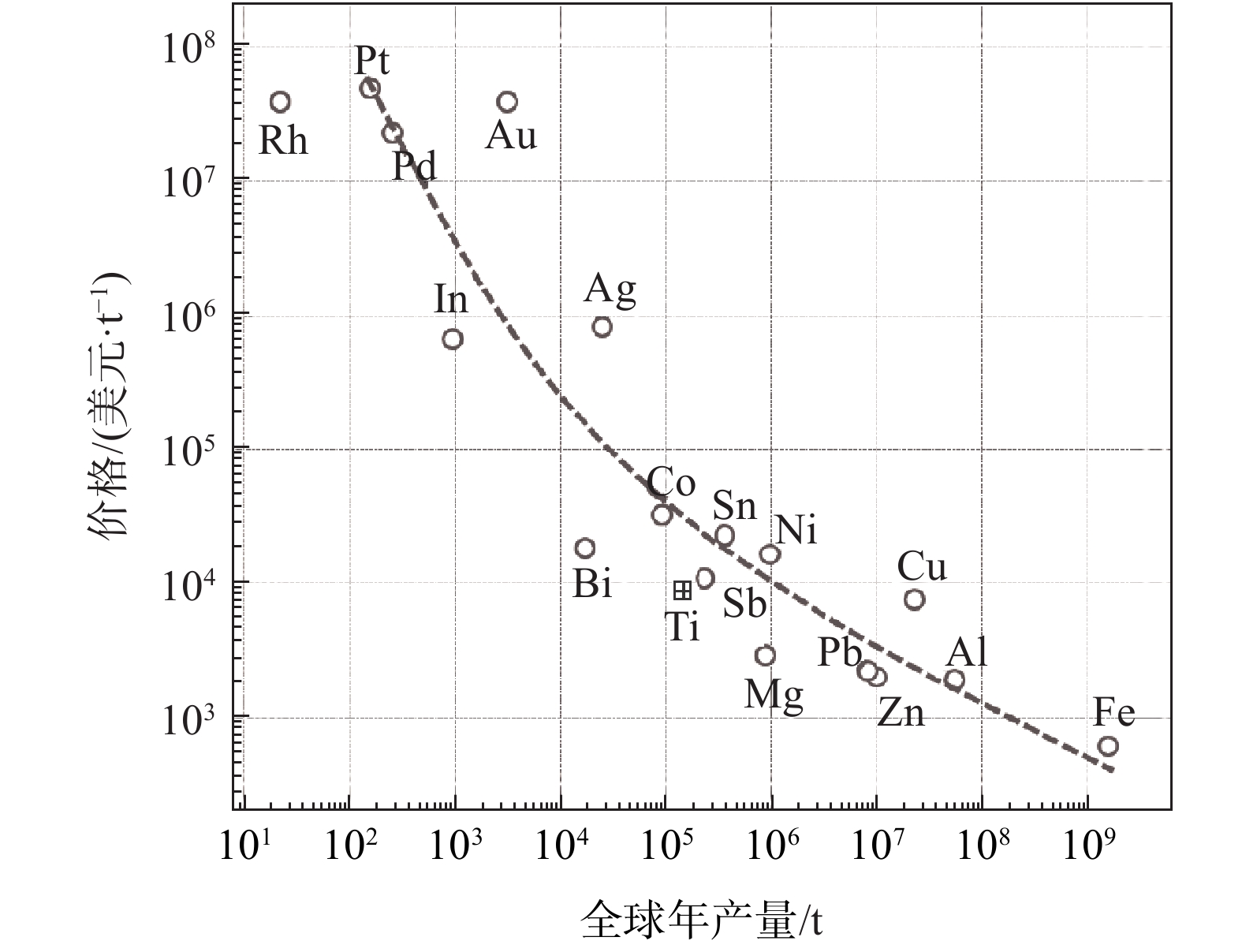
摘要: 金属钛由于其优异的性能而被用作高端结构材料。然而,在我们的日常生活中金属钛的利用却非常有限。全球金属钛年产量仅为钛白粉的1/30,从金属钛的性能和其丰富资源储量来看是极其不自然的。制约金属钛广泛使用的主要因素是其昂贵的价格。比较了金属钛、铝以及钢铁从矿石原料到金属的生产过程,并分析了现行金属钛生产过程的成本构成。在此基础上,分析了迄今为止已开发的新型钛冶炼工艺,从缩短生产流程,尤其是减少化学反应步骤的角度出发,探讨简化冶金流程、降低生产成本的可能性。指出,比较而言,以钛铁矿FeTiO3作为起始原料,经过碳热还原制备碳氧化钛TiCxO1−x,进而熔盐电解制备金属钛的冶金流程,有望大幅度降低能耗和生产成本。有待解决的问题是碳氧化钛阳极的规模化加工,以及在实际电解过程的连续运行等。Abstract: Titanium is currently used as an advanced structural material for its high strength, low density, and excellent corrosion resistance. However, in our daily lives, people are not familiar with titanium metal because we rarely use it. The worldwide annual production of titanium metal is less than 1/30 of that of titanium oxide (TiO2). This is very unnatural considering the excellent properties and abundant reserves of titanium. The limitation of titanium application derives from its high cost. In this paper, the metallurgical process of titanium was analyzed, and compared with the processes of iron and aluminum. The details of the production cost of the current metallurgical process were analyzed. New titanium metallurgical processes were reviewed, and the possibility of reducing the production cost was discussed. The energy consumption and operation cost of titanium metallurgical process will be remarkably reduced through the combination of the carbon thermoreduction and molten salt electrolysis, using ilmenite (FeTiO3) as the raw material. The main challenges for scaling up of the USTB process are preparation of large size anode TiCxO1−x and continuous operation of large size electrolysis cell.
-
Key words:
- titanium /
- production cost /
- TiO2 /
- ilmenite /
- TiCxO1−x /
- molten salt electrolysis
-
表 1 金属含量、世界年产量、矿石价格和金属价格
Table 1. Reserves (Clarke number, CN), world annual production (WAP), mining cost (MC), and price of metals
元素 金属含量(CN)
×106世界年产量
/万t矿石价格
/(美元· t−1)金属价格/(美元· t−1) Al 81300 5800 260 2 000 Fe 50000 162800 150 325 Mg 20900 101 245 2180 Ti 4400 20 460 8150 Zn 70 1400 2400 2860 Cu 55 2350 5090 5870 Pb 13 1110 2370 2720 -
[1] (东邦钛业有限公司[DB/OL]. https://www.toho-titanium.co.jp/products/sponge.html.)東邦チタニウム株式会社: https://www.toho-titanium.co.jp/products/sponge.html [2] Marco V. Ginatta, Gianmichele Orsello. Plant for the electrolytic production of reactive metals in molten salt baths, US Patent: 4670121[P]. 1987. [3] Marco V Ginatta, Gianmichele Orsello, Riccardo Berruti. Method and cell for the electrolytic production of a polyvalent metal, US Patent: 5015342[P]. 1991. [4] Marco V. Ginatta. Economics and production of primary titanium by electrolytic winning[C]//EPD Congress, 2001: 13−41. [5] Toshihide Takenaka, Takayuki Suzuki, Masahiro Ishikawa, et al. The new concept for electrowinning process of liquid titanium metal in molten salt[J]. Electrochemistry, 1999,67(6):661−668. doi: 10.5796/electrochemistry.67.661 [6] Cardarelli Francois. A method for electrowinning of titanium or alloy from titanium oxide containing compound in the liquid state, International Pat: WO 03046258[P]. 2003. [7] Donald R Sadoway. Electrochemical processing of refractory metal[J]. JOM, 1991,43(7):15−19. doi: 10.1007/BF03220614 [8] George Zheng Chen, Derek J Fray, Tom W Farthing. Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride[J]. Nature, 2000,407(6802):361−364. doi: 10.1038/35030069 [9] Derek J Fray. Emerging molten salt technologies for metals production[J]. JOM, 2001,53(10):27−31. [10] Katsutoshi Ono, Ryosuke O Suzuki. A new concept for producing Ti sponge: calciothermic reduction[J]. JOM, 2002,54(2):59−61. doi: 10.1007/BF02701078 [11] Ryosuke O Suzuki, Shuichi Inoue. Calciothermic reduction of titanium oxide in molten CaCl2[J]. Metallurgical and Materials Transactions B, 2003,34(3):277−285. doi: 10.1007/s11663-003-0073-2 [12] Toru H Okabe, Takashi Oda, Yoshitake Mitsuda. Titanium powder production by preform reduction process (PRP)[J]. Journal of Alloys and Compounds, 2004,364(1−2):156−163. doi: 10.1016/S0925-8388(03)00610-8 [13] Toru H Okabe, Takashi Oda, Yoshitake Mitsuda. Titanium powder production by preform reduction process[C]//Ti-2003 Science and Technology. In Lhutjering G, Albrecht J eds. Weinheim: Wiley-VCH, 2003: 261−268. [14] Fang Zhigang Zak, Xia Yang, Sun Pei, et al. Molten salt de-oxygenation of metal powders, International Pat: WO 2016090052[P]. 2016. [15] Zhang Ying, Fang Zhigang Zak, Xia Yang, et al. Hydrogen assisted magnesiothermic reduction of TiO2[J]. Chemical Engineering Journal, 2017,308:299−310. doi: 10.1016/j.cej.2016.09.066 [16] Hiroaki Okamoto, Mark Schlesinger. Binary alloy phase diagrams[M]. ASM International, Materials Park, OH, USA, 1990. [17] Eugene Wainer. Cleveland Heights. Production of titanium, US Patent: 2722509[P]. 1955. [18] Eugene Wainer. Cell feed material for the production of titanium, US Patent: 2868703[P]. 1959. [19] Takeuchi S, Watanabe H. Studies on the electrolytic extraction of Ti from TiO, TiC and TiCO alloy as anode[J]. Journal of the Japan Institute of Metals, 1964,28(10):627−632. (竹内栄, 渡辺治. 从TiO、TiC和TiCO合金阳极中电解提取Ti的研究[J]. 日本金属学院学报, 1964,28(10):627−632. doi: 10.2320/jinstmet1952.28.10_627 [20] Yasuhiko Hashimoto. Molten salt electrolysis of TiCO alloy or TiC as anode[J]. Journal of the Japan Institute of Metals, 1968,32(12):1327−1334. (桥本雍彦. 以TiCO合金或TiC为阳极的熔盐电解制备钛金属[J]. 日本金属学院学报, 1968,32(12):1327−1334. doi: 10.2320/jinstmet1952.32.12_1327 [21] Hashimoto Y. Extraction of Ti from arc-reduced Ti-CO and TiC soluble anodes[J]. Journal of the Japan Institute of Metals, 1971,35(3):282−288. (桥本雍彦. Ti-CO和TiC可溶性阳极电弧还原提取Ti[J]. 日本金属学院学报, 1971,35(3):282−288. doi: 10.2320/jinstmet1952.35.3_282 [22] Hashimoto Y. Anodic dissolution of low-grade (δ) Ti-C-O soluble anode in molten salt for electrolytic extraction of titanium[J]. Journal of the Japan Institute of Metals, 1971,35(5):480−486. (桥本雍彦. 从低品位Ti-C-O可溶性阳极中熔盐电解溶出钛[J]. 日本金属学院学报, 1971,35(5):480−486. doi: 10.2320/jinstmet1952.35.5_480 [23] (朱鸿民, 焦树强, 顾学范. 一氧化钛/碳化钛可溶性固溶体阳极电解生产纯钛的方法, 中国: 200510011684.6[P]. 2005.)Zhu Hongmin, Jiao Shuqiang, Gu Xuefan. A method for producing pure titanium through electrolysis of TiO·mTiC (0≤m≤1) soluble solid solution anode, Chinese Patent: CN200510011684.6[P]. 2005. [24] Jiao Shuqiang, Zhu Hongmin. Novel metallurgical process for titanium production[J]. Journal of Materials Research, 2006,21(9):2172−2175. doi: 10.1557/jmr.2006.0268 [25] Jiao Shuqiang, Ning Xiaohui, Huang Kai, et al. Electrochemical dissolution behavior of conductive TiCxO1−x solid solutions[J]. Pure and Applied Chemistry, 2010,82(8):1691−1699. doi: 10.1351/PAC-CON-09-10-39 [26] Gungor Mehmet N, M Ashraf Imam, Froes F H. Innovations in titanium technology[M]. Warendale: Wiley’s Publishing, 2007. [27] Withers J. International round table on titanium production in molten salts[C]//Cologne, Germany, 2008, 70: 2–4. [28] Ning Xiaohui, Xiao Jiusan, Jiao Shuqiang, et al. Anodic dissolution of titanium oxycarbide TiCxO1−x with different O/C ratio[J]. Journal of the Electrochemical Society, 2019,166(2):E22. doi: 10.1149/2.0141902jes [29] Gao Chengjun, Jiang Bo, Cao Zhanmin, et al. Preparation of titanium oxycarbide from various titanium raw materials: Part I. Carbothermal reduction[J]. Rare Metals, 2010,29(6):547−551. doi: 10.1007/s12598-010-0166-4 -





 下载:
下载: