Study on sodium roasting kinetics of vanadium removal slag of titanium tetrachloride
-
摘要: 基于非等温热重分析研究Na2CO3添加量和升温速率对含钒尾渣氧化的影响规律,采用Kissinger-Akahira-Sunose(KAS)法计算了含钒尾渣氧化过程活化能和指前因子,并通过Coats-Redfem法推断机理函数并建立不同阶段所适用的动力学方程。结果表明:含钒尾渣完全氧化的温度为700 ℃,随Na2CO3添加量增加,表观活化能逐渐降低,氧化速率提高;当Na2CO3添加量超过20%后,钒渣在氧化焙烧过程中出现玻璃相,产生烧结现象,表观活化能开始逐渐增大,氧化速率降低。钠化焙烧过程分为四个阶段,其动力学方程分别为:第一阶段二维扩散dα/dT=exp(−72.03/RT)4(1−α)1/2[1−(1−α)1/2]20.022/β,第二阶段三维扩散dα/dT=exp(−23.7/RT)3/2(1−α)4/3[(1−α)−1/3−1]−10.014/β,第三阶段化学反应dα/dT=exp(−27.91/RT) (1−α)20.06/β,第四阶段形核与长大dα/dT=exp(−12.09/RT)2(1−α)[−ln(1−α)]1/20.14/β。Abstract: Based on non-isothermal thermogravimetric analysis, the influences of Na2CO3 addition and heating rate on oxidation of vanadium removal slag of titanium tetrachloride (vanadium-containing tailings) were studied. The Kissinger-Akahira-Sunose (KAS) method was used to calculate the activation energy and pre-exponential factor of the oxidation process of vanadium-containing tailings. Through the Coats-Redfem method, the mechanism function was inferred and the kinetics equations of different stages were established. The results show that the temperature for complete oxidation of the vanadium-containing tailings is 700 ℃. With the increase of Na2CO3 addition, the apparent activation energy gradually decreases and the oxidation rate increases. While the Na2CO3 addition exceeds 20%, the glassy phase appears during the oxidation roasting process which results in sintering, and the apparent activation energy gradually increases with the oxidation rate decreased consequently. The sodium roasting process can be divided into four stages and the kinetics equations are as follows: the first stage of two-dimensional diffusion with dα/dT=exp(−72.03/RT)4(1−α)1/2[1−(1−α)1/2]20.022/β, the second stage of three-dimensional diffusion with dα/dT = exp (−23.7/RT)3/2(1−α)4/3[(1−α)−1/3−1]−10.014/β, the third stage of chemical reaction with dα/dT=exp(−27.91/RT)(1−α)20.06/β and the fourth stage of nucleation and growth with dα/dT=exp(−12.09/RT)2(1−α)[−ln(1−α)]1/20.14/β.
-
0. 引言
钒是一种重要资源,我国对含钒钢渣、含钒固废等提钒原料的开发和利用十分重视[1]。近年来,氯化法生产海绵钛和钛白粉工业发展迅速[2],该流程中钒将以VOCl3形式进入中间体四氯化钛中,降低其纯度,并影响最终产品的质量[3]。工业上一般通过有机物除钒工艺[4-5],获得精制四氯化钛和除钒尾渣。对于该钒尾渣中钒的提取,目前主要有钠化焙烧-水浸提钒[6-7]、钙化焙烧-酸浸提钒[8]、亚熔盐法提钒[9]等工艺,其原理都是将钒渣中的钒进行物相重构,将低价的钒转化为高价的水溶性钒酸钠或者酸溶性钒酸钙。因此,从技术方面来看,整个工艺流程中焙烧过程的物相重组效率关乎整个流程转化率,是提高钒回收率的关键。大量研究主要集中于对钠化焙烧过程物相变化和相应温度研究开展[10-13],而基于活化能、机理函数和指前因子的钒渣钠化焙烧非等温氧化动力学的研究较少。
笔者采用热重技术对不同Na2CO3添加量下钒渣氧化的热重特性进行研究,分析了Na2CO3添加量、升温速率等因素对钒渣氧化的影响规律,同时对钒渣氧化过程的表观活化能进行求解,推断出反应阶段的机理函数,并建立动力学方程。
1. 试验
1.1 试验原料
试验所用的四氯化钛除钒尾渣来自某工厂,其主要化学成分见表1。由分析结果可知,该尾渣中钒含量为11.17%,同时含有大量的氯、铁、钛、铝、锆以及少量的硅、铬等元素。试验过程所使用的Na2CO3试剂为分析纯,试验用水为去离子水。
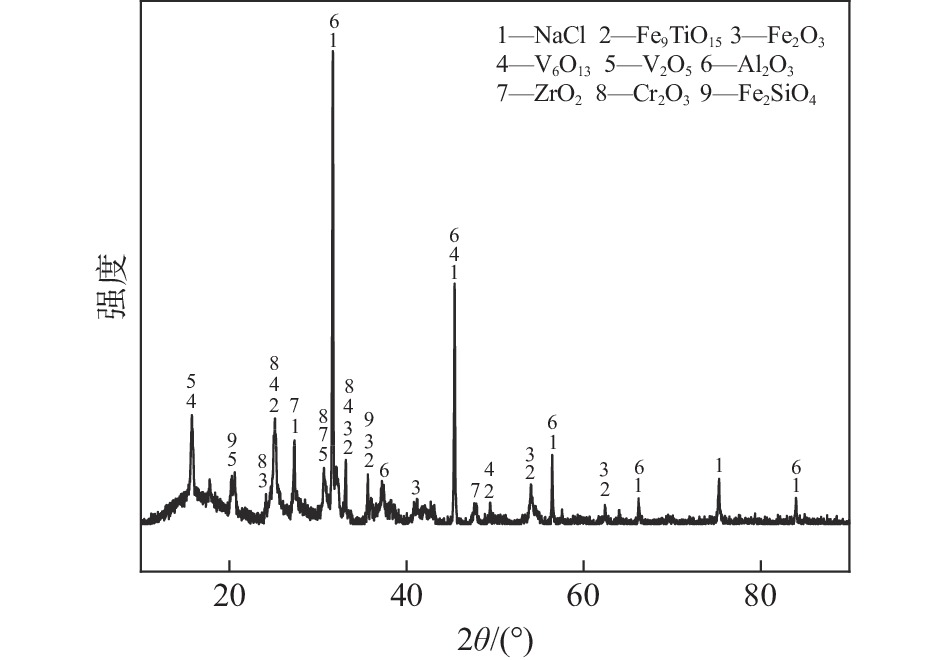
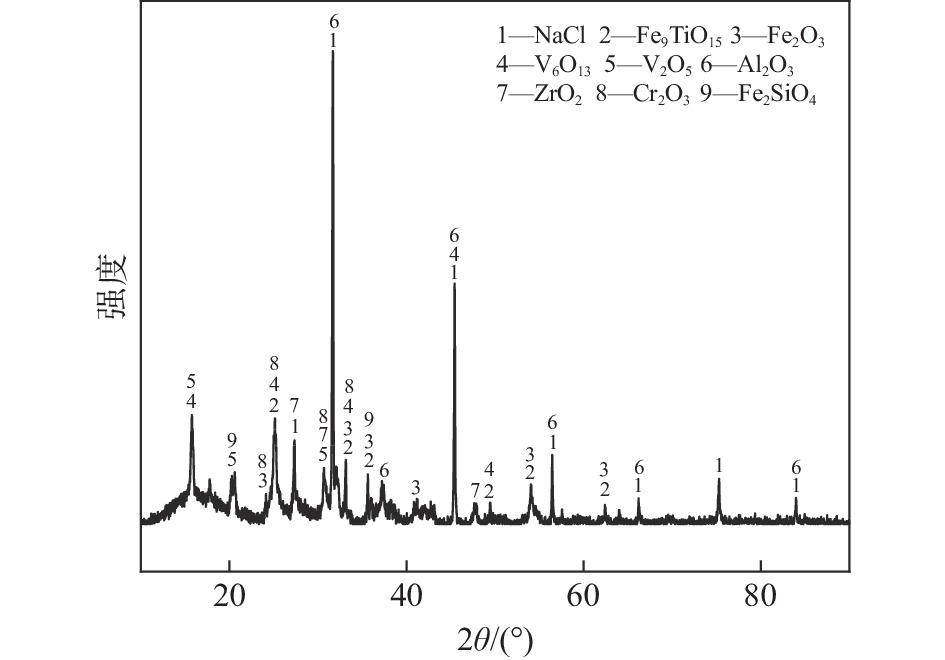
图1为试验所用四氯化钛除钒尾渣的XRD图谱,由图1可知尾渣中钒主要以V6O13和V2O5形式存在,同时含有NaCl、Fe2O3、Fe9TiO15、Al2O3、ZrO2、Fe2SiO4以及Cr2O3等组分。
表 1 粗四氯化钛精制尾渣的主要化学成分Table 1. Main chemical compositions of vanadium removal slag of crude titanium tetrachloride% Cl Fe2O3 TiO2 Al2O3 V2O5 ZrO2 C SiO2 Cr2O3 31.95 19.18 15.39 8.64 11.17 6.77 2.68 1.86 0.81 1.2 试验仪器
本试验所用设备有电热恒温干燥箱(长葛市唯恒机械设备有限公司)、EDX-7000型X射线荧光光谱分析仪(日本岛津公司)、Ultima IV型X射线衍射仪(日本理学株式会社)和D-09123热重差热分析仪(梅特勒-托利多)。
1.3 试验方法
热重试验过程:将四氯化钛除钒尾渣过100目(150 μm)筛后放入105 ℃干燥箱中,4 h后将干燥的除钒尾渣与一定质量的Na2CO3(10%~30%)充分混合制样,随后将样品(17 mg±0.5 mg)放入氧化铝坩埚中进行热重测试,试验过程氮气流速为20 mL/min,升温速率分别为10、15、20 K/min,加热终点温度为1300 ℃。
2. 结果与讨论
2.1 TG/DTG分析
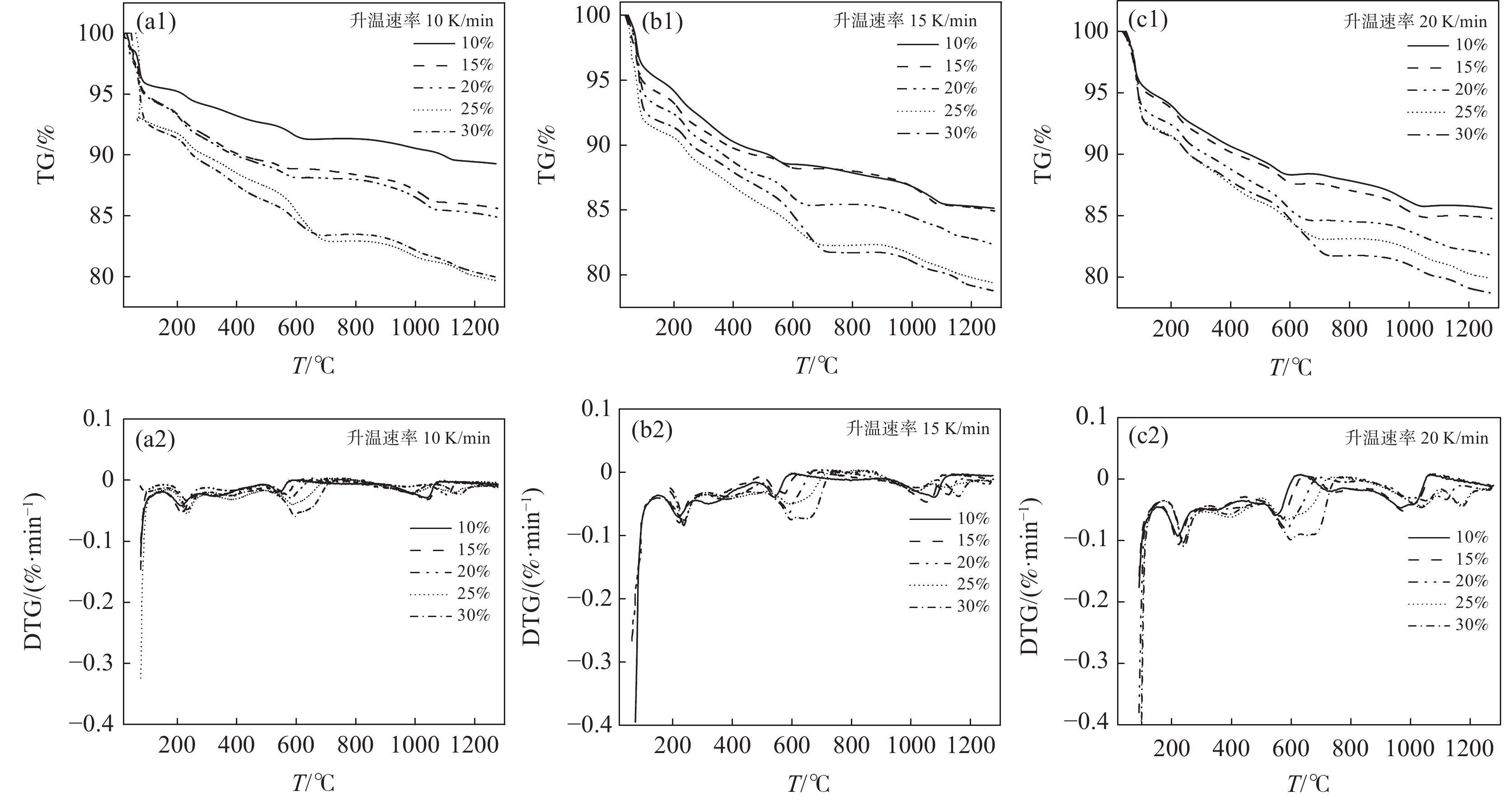
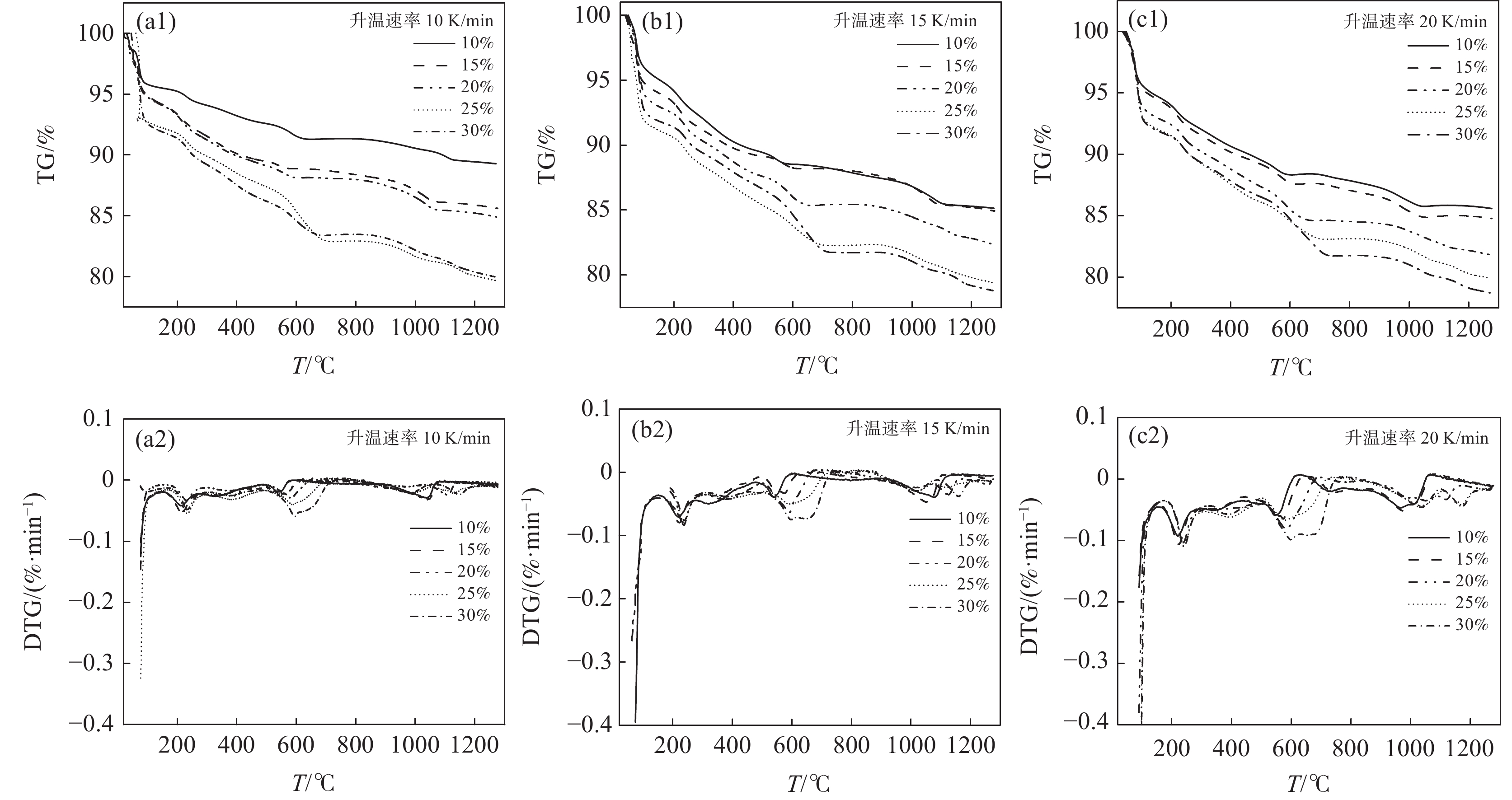
Na2CO3添加量为10%、15%、20%、25%、30%的钒渣在不同升温速率(10、15、20 K/min)下的TG/DTG曲线如图2所示。结果表明,初始阶段(0~130 ℃)随着温度的升高,由于结晶水蒸发而导致钒渣质量迅速下降,130 ℃以后,随着温度的继续上升,钒渣在氧化焙烧过程中开始发生化学反应,主要反应如式(1)~(5)所示,钒渣质量缓慢减少,在700 ℃开始趋于稳定,说明钒渣完全氧化的温度在700 ℃;不同Na2CO3添加量下的热重曲线区别较明显,在相同升温速率下,随着Na2CO3添加量的增大,减重速率逐渐加快。随着Na2CO3添加量增大,DTG曲线在600 ℃时可以看出明显向下偏移,这是因为钒渣中氧化物、尖晶石和橄榄石等物相氧化在热力学上存在明显的差异,随着Na2CO3添加量的增大,钒渣中各个物相反应的更加充分,进而使各个物相反应的热力学条件发挥完全,各反应的竞争作用逐渐减弱。当升温速率由10 K/min增大到20 K/min时,各特征峰的峰值温度均向后推移,这主要是由于升温速率的升高引起的热滞后现象所导致[14]。
$$ \begin{split} & \rm 9Fe_{9}TiO_{15}+Na_{2}CO_{3}+2.25O_{2}=\\ & \rm Na_{2}Ti_{9}O_{19}+40.5Fe_{2}O_{3}+CO_{2 } \end{split}$$ (1) $$ \rm V_{6}O_{13}+O_{2}=3V_{2}O_{5 }$$ (2) $$ \rm V_{2}O_{5}+Na_{2}CO_{3}=2NaVO_{3}+CO_{2 } $$ (3) $$ \begin{split} & \rm 2Fe_{2}SiO_{4}+2Na_{2}CO_{3}+O_{2}=\\ & \rm 2Fe_{2}O_{3}+2Na_{2}SiO_{3}+2CO_{2 } \end{split} $$ (4) $$ \rm Cr_{2}O_{3}+1.5O_{2}+2Na_{2}CO_{3}=2Na_{2}CrO_{4}+2CO_{2 }$$ (5) 2.2 表观活化能分析
通常表观活化能计算的方法有三种,分别为Flynn-Wall-Ozawa(FWO)法[15]、Friedman[16]法和Kissinger-Akahira-Sunose(KAS)[17]法,相比而言,其中KAS法在温度积分的近似误差较小,计算出的活化能更加精确[18],所以笔者将使用KAS法对钒渣非等温热分析的活化能进行求解,公式如(6)所示:
$$ \mathrm{ln}\left(\frac{\beta }{{T}_{\alpha }^{2}}\right)=-\left(\frac{{E}_{\alpha }}{\mathrm{R}{T}_{\alpha }}\right)+\mathrm{l}\mathrm{n}\frac{A\mathrm{R}}{{E}_{\alpha }G\left(\alpha \right)} $$ (6) 式中,
$ {T}_{\alpha } $ 为指定α值对应的热力学温度,K;β为升温速率,K/min;A为指前因子;$ {E}_{\alpha } $ 为指定α值对应的活化能,kJ/mol;G(α)为动力学模式函数的积分形式;R为气体常数,8.314 J/(mol·K)。在不同的升温速率下取相同的值对应的温度$ {T}_{\alpha } $ ,作ln(β/T2)−1/T图,并进行多元线性回归分析,其中直线斜率为-$ {E}_{\alpha } $ /R,即可求出相应α对应的活化能$ {E}_{\alpha } $ 。转化率α定义为:
$$ \alpha =\frac{{m}_{0}-{m}_{t}}{{m}_{0}-{m}_{f}}=\frac{\Delta {m}_{t}}{\Delta {m}_{{\rm{max}}}} $$ (7) 式中,
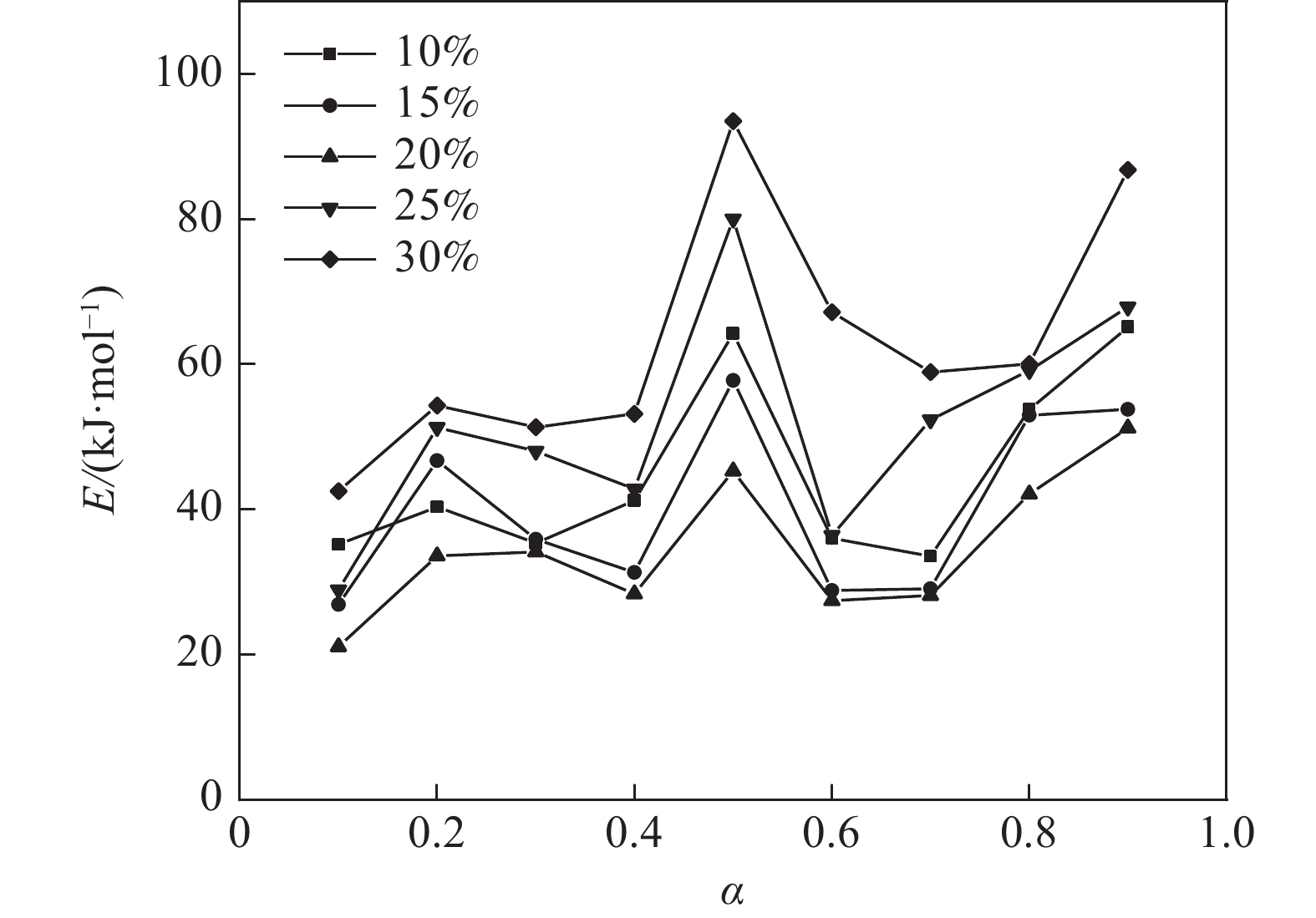
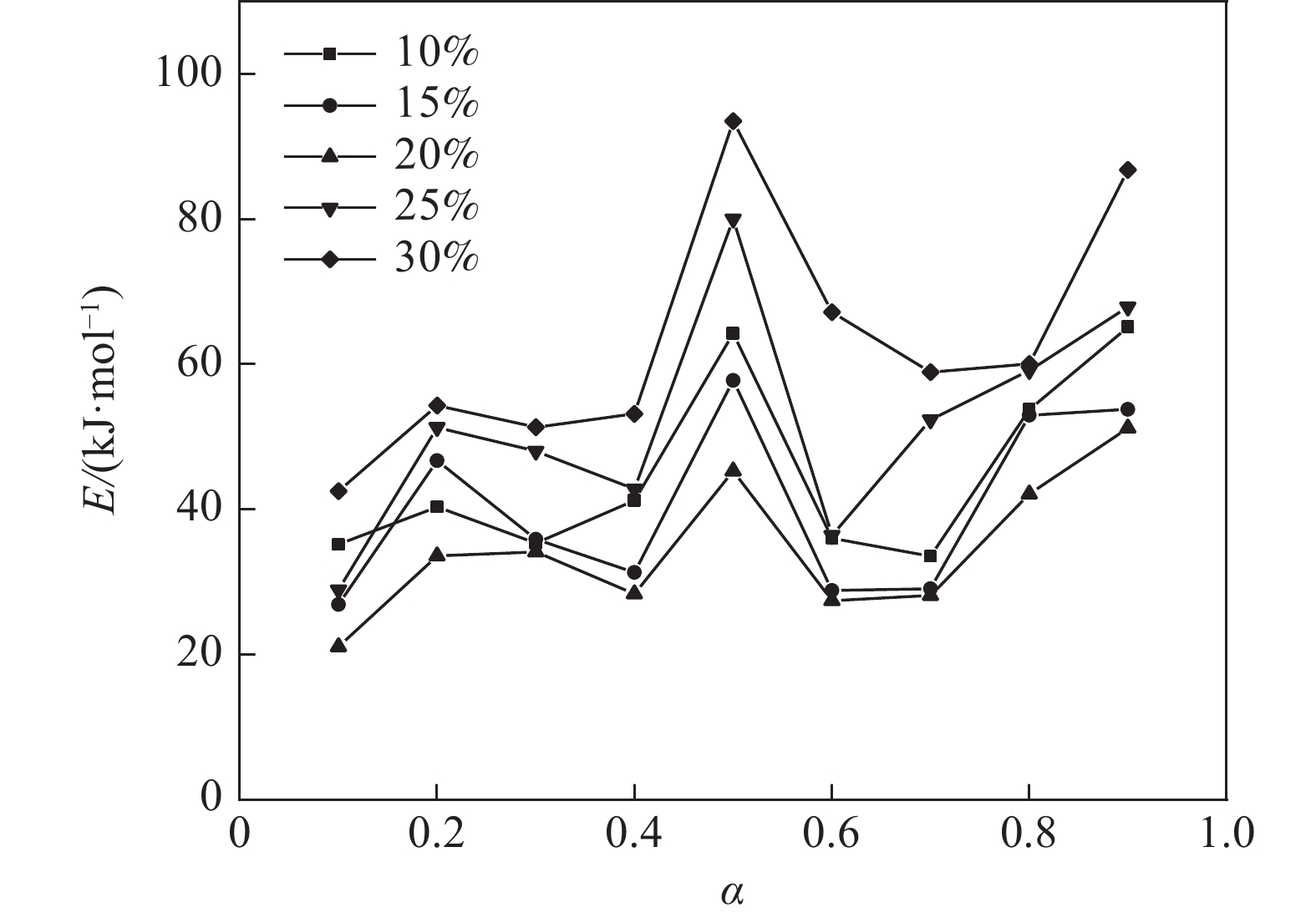
$ {m}_{0} $ 为样品的初始质量,mg;$ {m}_{t} $ 为t时刻对应的样品重量,mg;$ {m}_{f} $ 为最后时刻样品的重量,mg;$ \Delta {m}_{t} $ 为$ t $ 时刻对应的样品增重率,%;$ \Delta {m}_{\mathrm{m}\mathrm{a}\mathrm{x}} $ 为样品的最大增重率,%。不同Na2CO3添加量下表观活化能与转换率的关系如图3所示。由图3可知,随着Na2CO3添加量的增加,表观活化能逐渐降低,当Na2CO3添加量为20%时,钒渣氧化的最大表观活化能降低到了45.3 kJ/mol,继续增加Na2CO3至30%,导致钒渣在氧化焙烧过程中出现玻璃相,产生烧结现象,最大表观活化能上升到了93.5 kJ/mol。因此在小于20%Na2CO3添加量范围内,增大Na2CO3添加量可以加快钒渣的反应速率。焙烧过程中转化率和表观活化能呈现非线性变化,表明需用不同的动力学机理函数对钒渣焙烧动力学机制进行分析。
2.3 动力学机制分析
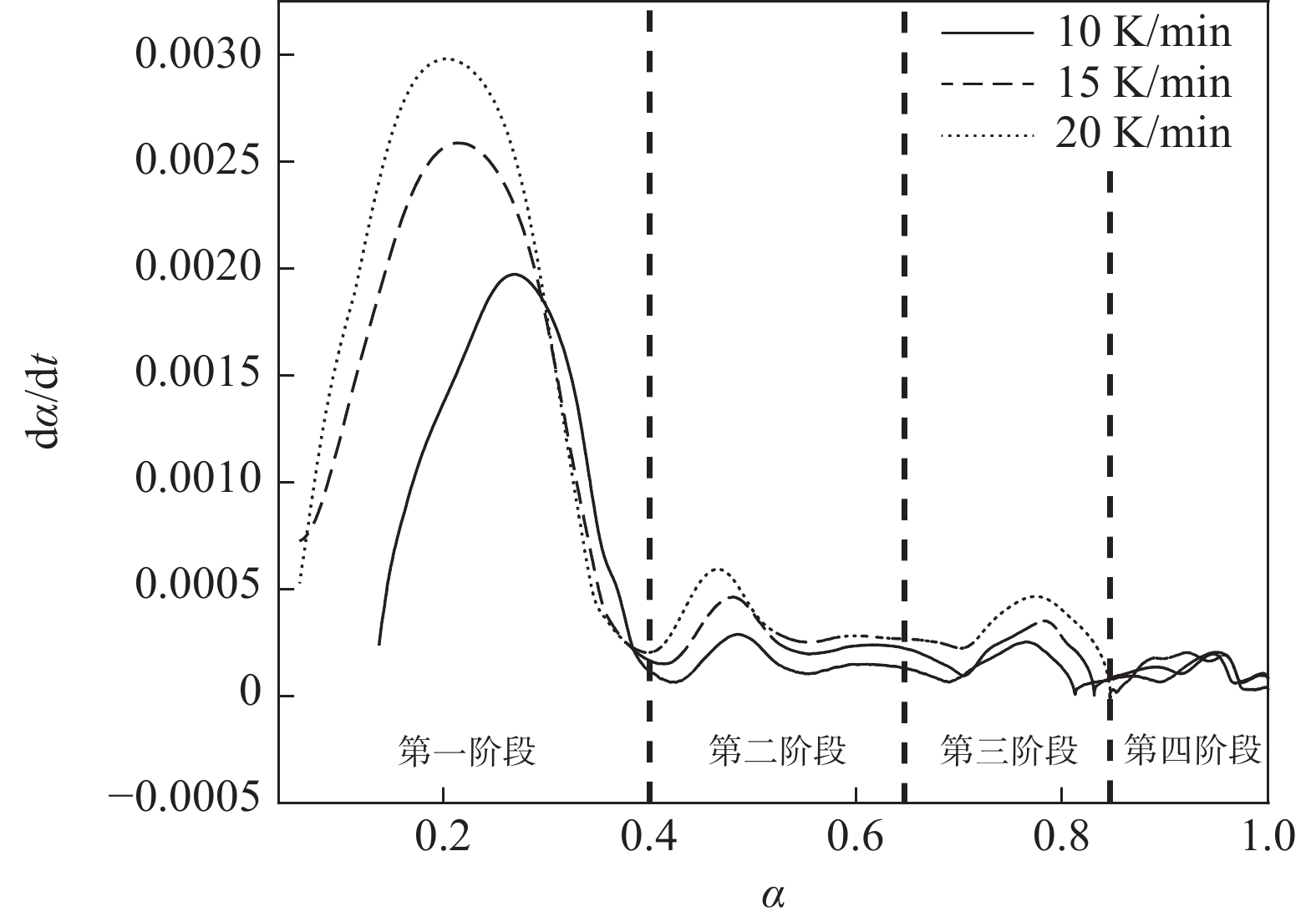
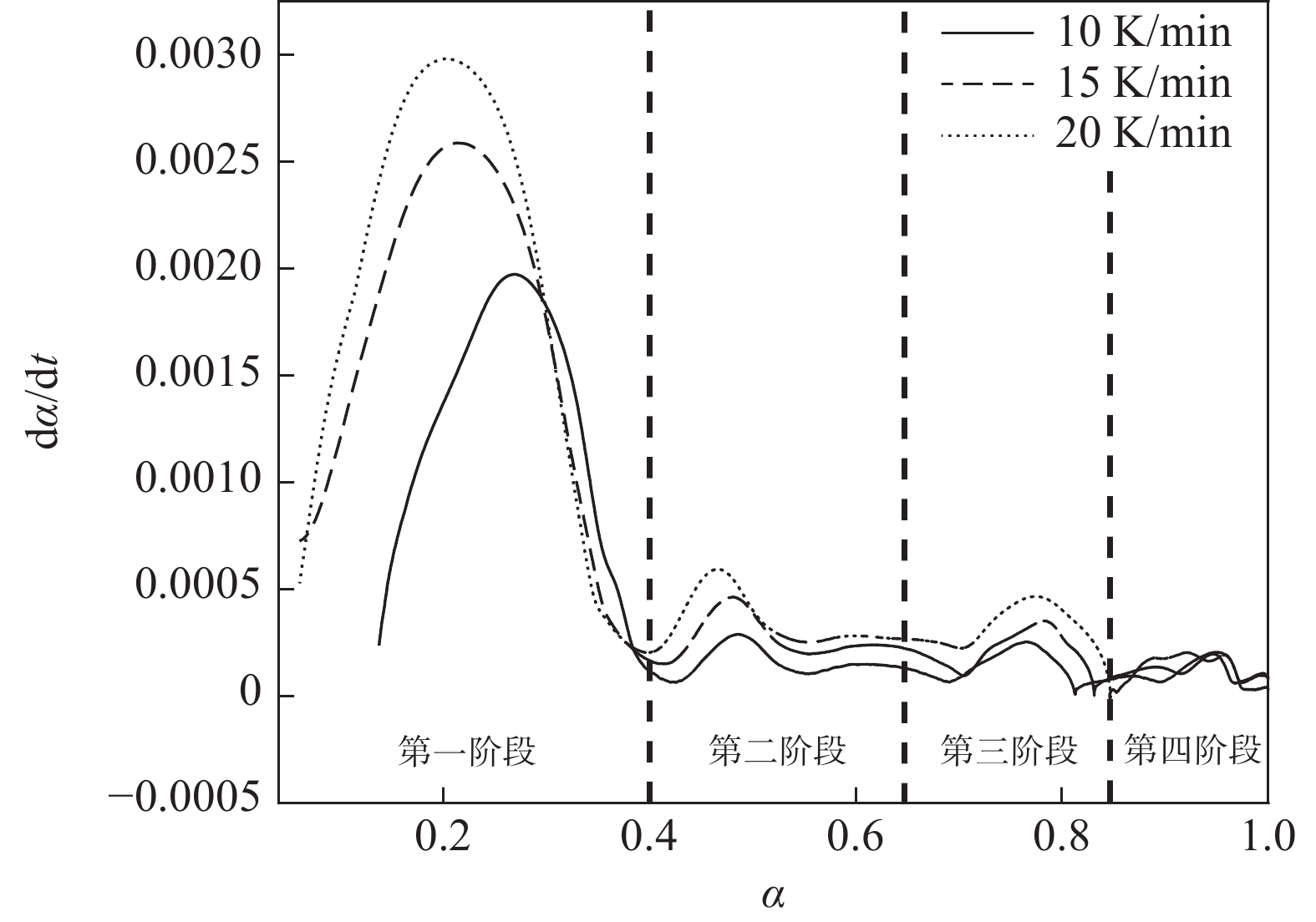
图4为四氯化钛除钒尾渣添加20%Na2CO3钠化焙烧反应速率与转化率α的曲线。从图4可以看出除钒尾渣钠化焙烧过程可以分为四个阶段,α取值分别为0~0.4、0.4~0.65、0.65~0.85和0.85~1.0。
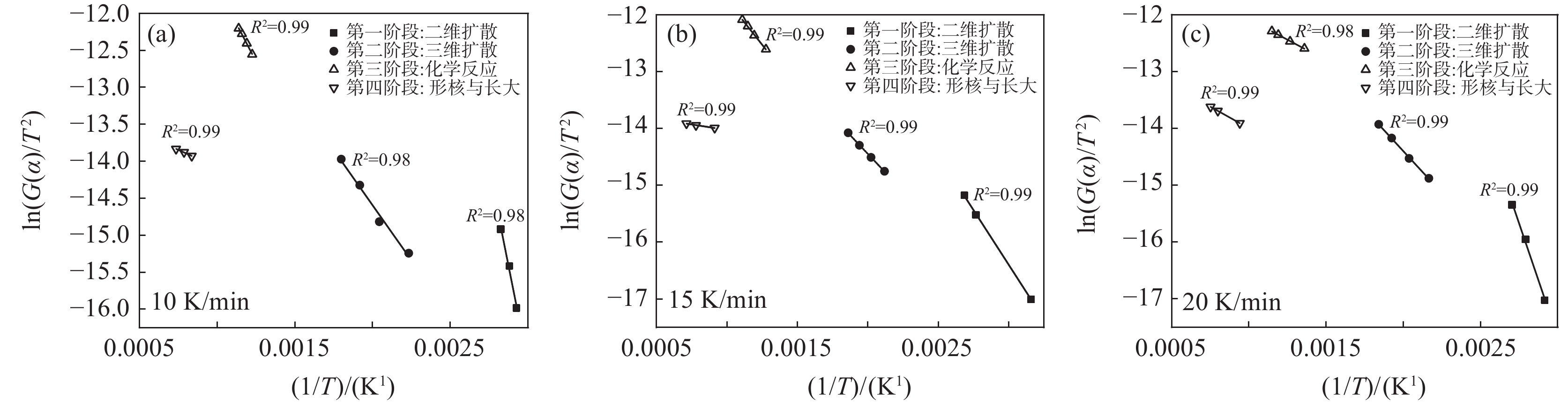
利用Coats-Redfem[19]法推断最概然机理函数,方程如式(8)所示,将文献[20]中标准动力学机理函数带入式(8),由ln(G(α)/T2)对1/T作图,采用最小二乘法线性回归,根据拟合直线的拟合度R2推测出最概然机理函数,结果如图5所示,可见各阶段与模式函数匹配的很好,拟合度均大于0.98。
$$ \mathrm{ln}\left(\frac{G\left(\alpha \right)}{{T}^{2}}\right)=\mathrm{ln}\left(\frac{AE}{\beta \mathrm{R}}\right)-\frac{E}{\mathrm{R}T} $$ (8) 除钒尾渣的钠化焙烧过程可分为四个阶段,第一阶段(α=0~0.4),符合二维扩散模型,其机理函数表达式为G(α)=[1−(1−α)1/2]2。第二阶段(α=0.4~0.65),符合三维扩散模型,其机理函数表达式为G(α)=[(1−α)−1/3−1]2。第三阶段(α=0.65~0.85),符合化学反应模型,其机理函数表达式为G(α)=(1−α)−1−1。第四阶段(α=0.85~1.0),符合形核与长大模型,其机理函数表达式为G(α)=[−ln(1−α)]1/2。
反应动力学方程通式可用式(9)表示:
$$ \frac{\mathrm{d}\alpha }{\mathrm{d}t}=kf\left(\alpha \right) $$ (9) f(α)为动力学模式函数的微分形式;k为反应速率常数(min−1),k=Aexp(−E/RT)。
升温速率β为常数,β=dT/dt,则式(9)可写为:
$$ \frac{\mathrm{d}\alpha }{\mathrm{d}T}=\frac{A}{\beta }\mathrm{e}\mathrm{x}\mathrm{p}\left(-\frac{E}{\mathrm{R}T}\right)f\left(\alpha \right) $$ (10) 由式(8)和图5可知,根据拟合直线的斜率可以求出不同阶段的表观活化能E,指前因子A由截距求出,结果见表2。
因此,第一阶段二维扩散反应动力学方程为:
$$ \frac{\mathrm{d}\alpha }{\mathrm{d}T}=0.022\frac{1}{\beta }\mathrm{e}\mathrm{x}\mathrm{p}\left(-\frac{72.03}{\mathrm{R}T}\right)4{\left(1-\alpha \right)}^{\frac{1}{2}}{[1-{(1-\alpha )}^{\frac{1}{2}}]}^{2} $$ (11) 第二阶段三维扩散的反应动力学方程为:
$$ \frac{\mathrm{d}\alpha }{\mathrm{d}T}=0.014\frac{1}{\beta }\mathrm{e}\mathrm{x}\mathrm{p}\left(-\frac{23.7}{\mathrm{R}T}\right)\frac{3}{2}{(1-\alpha )}^{\frac{4}{3}}{[{\left(1-\alpha \right)}^{-\frac{1}{3}}-1]}^{-1} $$ (12) 第三阶段化学反应的反应动力学方程为:
$$ \frac{\mathrm{d}\alpha }{\mathrm{d}T}=0.06\frac{1}{\beta }\mathrm{e}\mathrm{x}\mathrm{p}\left(-\frac{27.91}{\mathrm{R}T}\right){\left(1-\alpha \right)}^{2} $$ (13) 第四阶段形核与长大的反应动力学方程为:
$$ \frac{\mathrm{d}\alpha }{\mathrm{d}T}=0.14\frac{1}{\beta }\mathrm{e}\mathrm{x}\mathrm{p}\left(-\frac{12.09}{\mathrm{R}T}\right)2(1-\alpha ){[-\mathrm{l}\mathrm{n}(1-\alpha \left)\right]}^{\frac{1}{2}} $$ (14) 表 2 四氯化钛除钒尾渣添加20%Na2CO3钠化焙烧在不同阶段的表观活化能和指前因子Table 2. Apparent activation energy and pre-exponential factor in different stages for sodium roasting of vanadium removal slag of titanium tetrachloride with 20% Na2CO3阶段 不同升温速率时的活化能 活化能/(kJ·mol−1) 指前因子/min−1 10 K/min 15 K/min 20 K/min 活化能/(kJ·mol−1) 拟合度 活化能/(kJ·mol−1) 拟合度 活化能/(kJ·mol−1) 拟合度 第一阶段 77.66 0.99 71.98 0.99 66.44 0.99 72.03 0.022 第二阶段 24.81 0.99 21.78 0.99 24.51 0.98 23.7 0.014 第三阶段 33.67 0.98 26.04 0.99 24.03 0.99 27.91 0.06 第四阶段 13.43 0.98 10.21 0.99 12.64 0.99 12.09 0.14 3. 结论
1)通过热重分析可知,钒渣完全氧化的温度在700 ℃,并且随着Na2CO3添加量的增大,氧化过程各个反应的竞争作用逐渐减弱,减重速率逐渐加快。随着升温速率的升高,各特征峰峰值温度向后推移。
2)随着Na2CO3添加量的增加,表观活化能逐渐降低。当Na2CO3添加量增加至20%时,钒渣氧化的最大表观活化能降低到了45.3 kJ/mol,继续增加Na2CO3添加量到30%,钒渣氧化的最大表观活化能上升到了93.5 kJ/mol。
3)除钒尾渣的钠化焙烧过程可分为四个阶段:分别为α=0~0.4、α=0.4~0.65、α=0.65~0.85、α=0.85~1.0。第一阶段符合二维扩散模型,其动力学方程为
$$ \frac{\mathrm{d}\alpha }{\mathrm{d}T}=0.022\frac{1}{\beta }\mathrm{e}\mathrm{x}\mathrm{p}\left(-\frac{72.03}{\mathrm{R}T}\right)4{\left(1-\alpha \right)}^{\frac{1}{2}}{[1-{(1-\alpha )}^{\frac{1}{2}}]}^{2} $$ 第二阶段符合三维扩散模型,其动力学方程为
$$ \frac{\mathrm{d}\alpha }{\mathrm{d}T}=0.014\frac{1}{\beta }\mathrm{e}\mathrm{x}\mathrm{p}\left(-\frac{23.7}{\mathrm{R}T}\right)\frac{3}{2}{(1-\alpha )}^{\frac{4}{3}}{[{\left(1-\alpha \right)}^{-\frac{1}{3}}-1]}^{-1}$$ 第三阶段符合化学反应模型,其动力学方程为
$$ \frac{\mathrm{d}\alpha }{\mathrm{d}T}=0.06\frac{1}{\beta }\mathrm{e}\mathrm{x}\mathrm{p}\left(-\frac{27.91}{\mathrm{R}T}\right){\left(1-\alpha \right)}^{2} $$ 第四阶段符合形核与长大模型,其动力学方程为
$$ \frac{\mathrm{d}\alpha }{\mathrm{d}T}=0.14\frac{1}{\beta }\mathrm{e}\mathrm{x}\mathrm{p}\left(-\frac{12.09}{\mathrm{R}T}\right)2(1-\alpha ){[-\mathrm{l}\mathrm{n}(1-\alpha \left)\right]}^{\frac{1}{2}} $$ -
表 1 粗四氯化钛精制尾渣的主要化学成分
Table 1. Main chemical compositions of vanadium removal slag of crude titanium tetrachloride
% Cl Fe2O3 TiO2 Al2O3 V2O5 ZrO2 C SiO2 Cr2O3 31.95 19.18 15.39 8.64 11.17 6.77 2.68 1.86 0.81 表 2 四氯化钛除钒尾渣添加20%Na2CO3钠化焙烧在不同阶段的表观活化能和指前因子
Table 2. Apparent activation energy and pre-exponential factor in different stages for sodium roasting of vanadium removal slag of titanium tetrachloride with 20% Na2CO3
阶段 不同升温速率时的活化能 活化能/(kJ·mol−1) 指前因子/min−1 10 K/min 15 K/min 20 K/min 活化能/(kJ·mol−1) 拟合度 活化能/(kJ·mol−1) 拟合度 活化能/(kJ·mol−1) 拟合度 第一阶段 77.66 0.99 71.98 0.99 66.44 0.99 72.03 0.022 第二阶段 24.81 0.99 21.78 0.99 24.51 0.98 23.7 0.014 第三阶段 33.67 0.98 26.04 0.99 24.03 0.99 27.91 0.06 第四阶段 13.43 0.98 10.21 0.99 12.64 0.99 12.09 0.14 -
[1] Qu Jinwei, Zhang Ting′an, Niu Liping, et al. Technical progress of comprehensive utilization of converter vanadium slag[J]. Iron Steel Vanadium Titanium, 2020,41(5):1−7. (瞿金为, 张廷安, 牛丽萍, 等. 转炉钒渣的综合利用技术进展[J]. 钢铁钒钛, 2020,41(5):1−7. [2] Xie Qichun. Research and application of reclaiming ilmenite from titanium tailings in Panxi[J]. Mining and Metallurgical Engineering, 2018,38(3):40−42. (谢琪春. 攀西选钛尾矿中再回收钛铁矿工艺研究与应用[J]. 矿冶工程, 2018,38(3):40−42. doi: 10.3969/j.issn.0253-6099.2018.03.009 [3] Li Liang, Zhou Li, Li Dongqin, et al. Research on the recovery and utilization of TiCl4 refined tailings[J]. Iron Steel Vanadium Titanium, 2016,7(5):76−79. (李良, 周丽, 李冬勤, 等. TiCl4精制尾渣的回收利用研究[J]. 钢铁钒钛, 2016,7(5):76−79. [4] Zhou Li. Study on the vanadium removal process of the organic pretreatment of high vanadium content crude titanium tetrachloride[J]. Iron Steel Vanadium Titanium, 2017,38(4):24−28. (周丽. 高含钒粗四氯化钛有机物预处理除钒工艺研究[J]. 钢铁钒钛, 2017,38(4):24−28. doi: 10.7513/j.issn.1004-7638.2017.04.005 [5] Yu Jing, Zhang Ping, Chen Tianxiang, et al. Research on the process of removing vanadium from crude titanium tetrachloride organics[J]. Journal of Guizhou University of Technology(Natural Science Edition), 2008,37(2):29−32. (于静, 章平, 陈天祥, 等. 粗四氯化钛有机物除钒工艺研究[J]. 贵州工业大学学报(自然科学版), 2008,37(2):29−32. [6] Shi Zhixin. Characterization of the variation law of vanadium spinel and fayalite during the sodium roasting of vanadium slag[J]. Non-ferrous Metals (Mineral Processing Part), 2018,(4):4−8,14. (史志新. 钒渣钠化焙烧过程中钒尖晶石和铁橄榄石的变化规律表征[J]. 有色金属(选矿部分), 2018,(4):4−8,14. [7] Zhang Xinxia. Optimization of sodium roasting process for high silicon high calcium vanadium slag[J]. Ferro Alloys, 2013,44(1):22−24,29. (张新霞. 高硅高钙钒渣钠化焙烧工艺的优化研究[J]. 铁合金, 2013,44(1):22−24,29. doi: 10.3969/j.issn.1001-1943.2013.01.006 [8] Yang Z, Li H Y, Yin X C, et al. Leaching kinetics of calcification roasted vanadium slag with high CaO content by sulfuric acid[J]. International Journal of Mineral Processing, 2014,133:105−111. doi: 10.1016/j.minpro.2014.10.011 [9] Pan Ziwei, Zheng Shili, Wang Zhongxing, et al. High-efficiency simultaneous extraction process of vanadium and chromium from high chromium vanadium slag by sub-molten salt method[J]. Iron Steel Vanadium Titanium, 2014,35(2):1−8. (潘自维, 郑诗礼, 王中行, 等. 亚熔盐法高铬钒渣钒铬高效同步提取工艺研究[J]. 钢铁钒钛, 2014,35(2):1−8. doi: 10.7513/j.issn.1004-7638.2014.02.001 [10] Gao Jian, Liu Xibin, Shi Zhixin. Phase changes and vanadium element migration characteristics of vanadium slag during sodium oxidation roasting[J]. Mining and Metallurgy, 2019,28(3):105−110. (高健, 刘希斌, 史志新. 钒渣氧化钠化焙烧过程中物相变化及钒元素迁移特征[J]. 矿冶, 2019,28(3):105−110. doi: 10.3969/j.issn.1005-7854.2019.03.022 [11] Li Xinsheng, Xie Bing, Wang Guang, en, et al. Oxidation process of low-grade vanadium slag in presence of Na2CO3[J]. Transactions of Nonferrous Metals Society of China, 2011,21(8):1860−1867. doi: 10.1016/S1003-6326(11)60942-4 [12] Xie Zhaoming, Deng Rongrui, Liu Zuohua, et al. Evolutionary behavior of fractal growth of vanadium slag powder in sodium roasting converter[J]. Journal of Chemical Industry, 2019,70(5):1904−1912. (谢昭明, 邓容锐, 刘作华, 等. 钠化焙烧转炉钒渣粉体分形生长的演化行为[J]. 化工学报, 2019,70(5):1904−1912. [13] Wang Minghua, Zhao Hui, Liu Yan, et al. Semi-quantitative analysis of the sodiumization roasting process of vanadium slag[J]. Iron Steel Vanadium Titanium, 2017,38(5):31−36. (王明华, 赵辉, 刘岩, 等. 钒渣钠化焙烧过程的半定量分析[J]. 钢铁钒钛, 2017,38(5):31−36. doi: 10.7513/j.issn.1004-7638.2017.05.006 [14] Lu X L, Zhu Q, Meng Y Z. Kinetic analysis of thermal decomposition of poly (propylene carbonate)[J]. Polymer Degradation and Stability, 2005,89(2):282−288. doi: 10.1016/j.polymdegradstab.2004.12.025 [15] Flynn J H, Wall L A. A quick, direct method for the determination of activation energy from thermogravimetric data[J]. Journal of Polymer Science Part B:Polymer Letters, 1966,4(5):323−328. doi: 10.1002/pol.1966.110040504 [16] Criado J M, Sánchez-Jiménez P E, Pérez-Maqueda L A. Critical study of the isoconversional methods of kinetic analysis[J]. Journal of Thermal Analysis & Calorimetry, 2008,92(1):199−203. [17] Kissinger H E. Reaction kinetics in differential thermal analysis[J]. Analytical Chemistry, 1957,29(11):1702−1706. doi: 10.1021/ac60131a045 [18] Vyazovkin S, Chrissafis K, Lorenzo M L D, et al. ICTAC kinetics committee recommendations for collecting experimental thermal analysis data for kinetic computations[J]. Thermochimica ACTA, 2014,590:1−23. doi: 10.1016/j.tca.2014.05.036 [19] Coats A W, Redfern J P. Kinetic parameters from thermogravimetric data. II[J]. Nature, 1964,201:68−69. doi: 10.1038/201068a0 [20] Huang L, Chen Y C, Liu G, et al. Non-isothermal pyrolysis characteristics of giant reed using thermogravimetric analysis[J]. Energy, 2015,87:31−40. doi: 10.1016/j.energy.2015.04.089 -
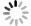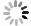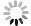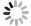




 下载:
下载:


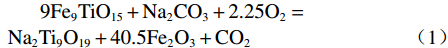

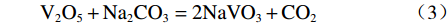
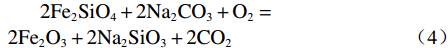
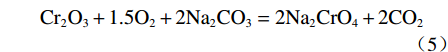

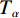
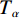
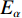
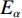
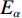

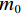
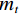
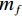
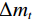

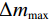



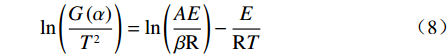


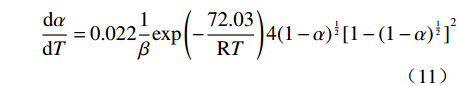

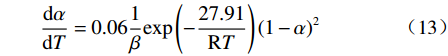
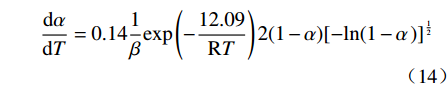
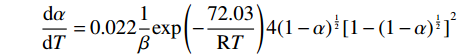
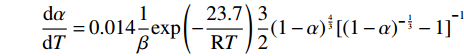
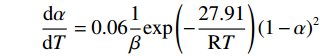
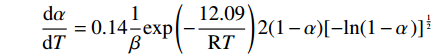
 下载:
下载: