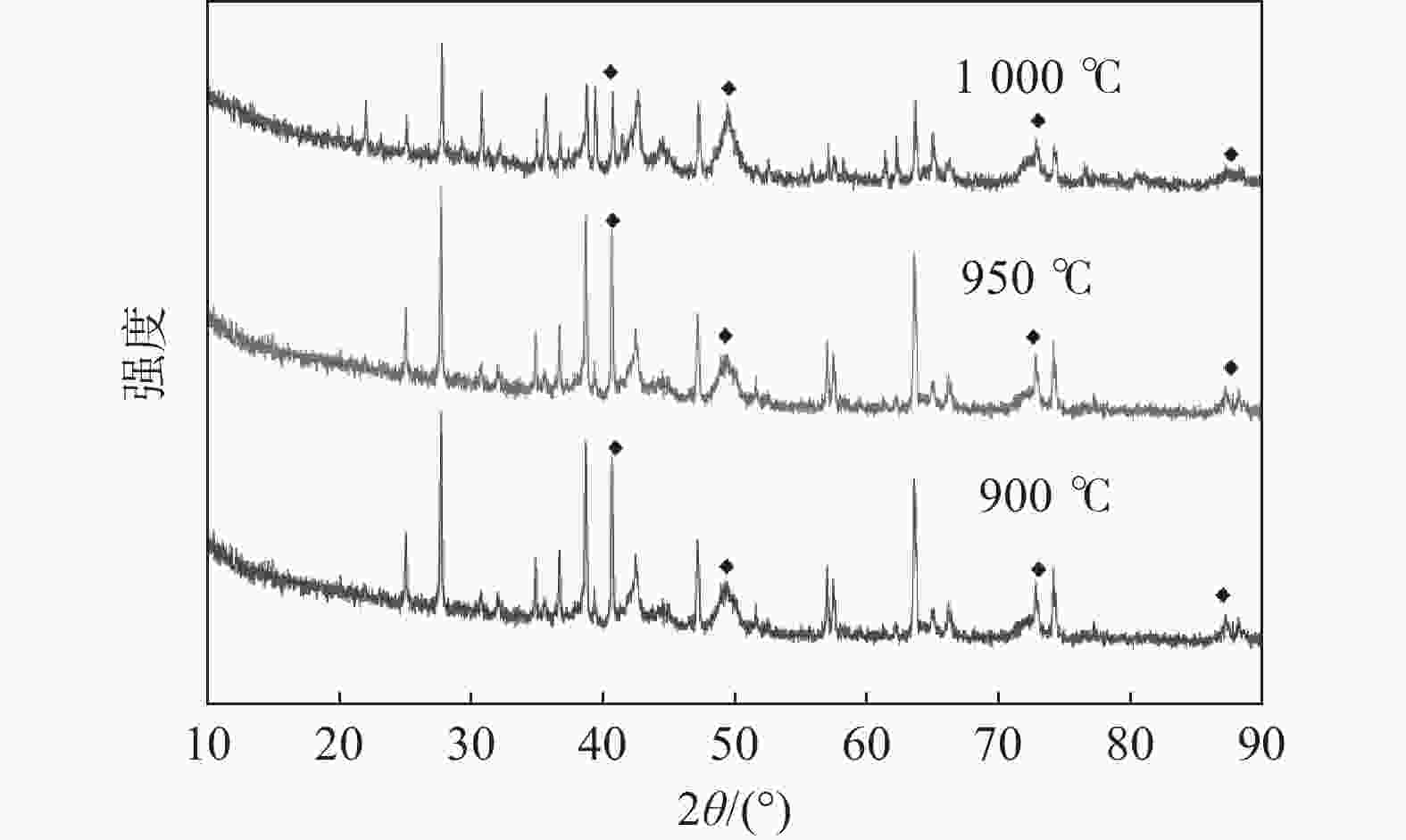
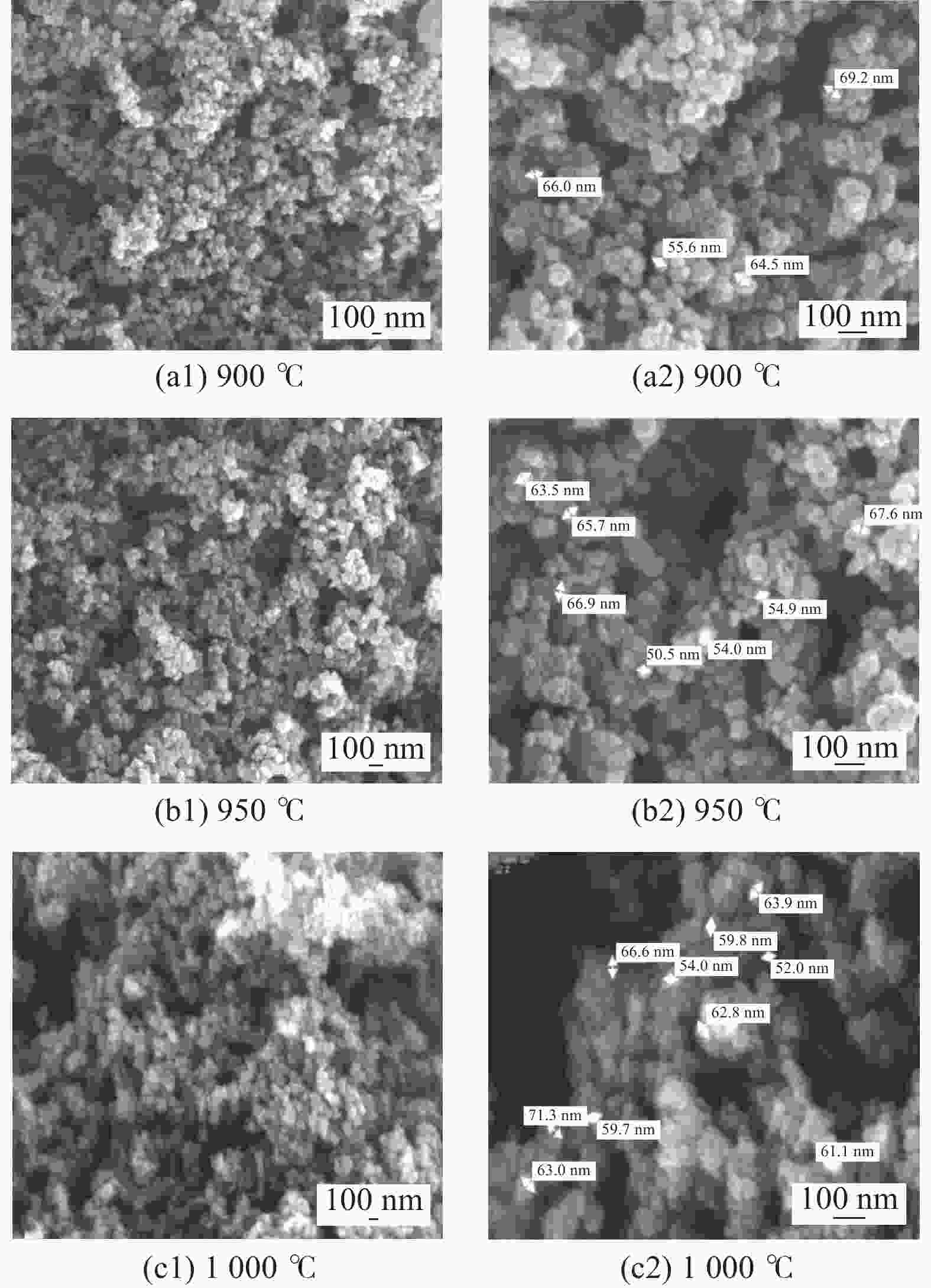
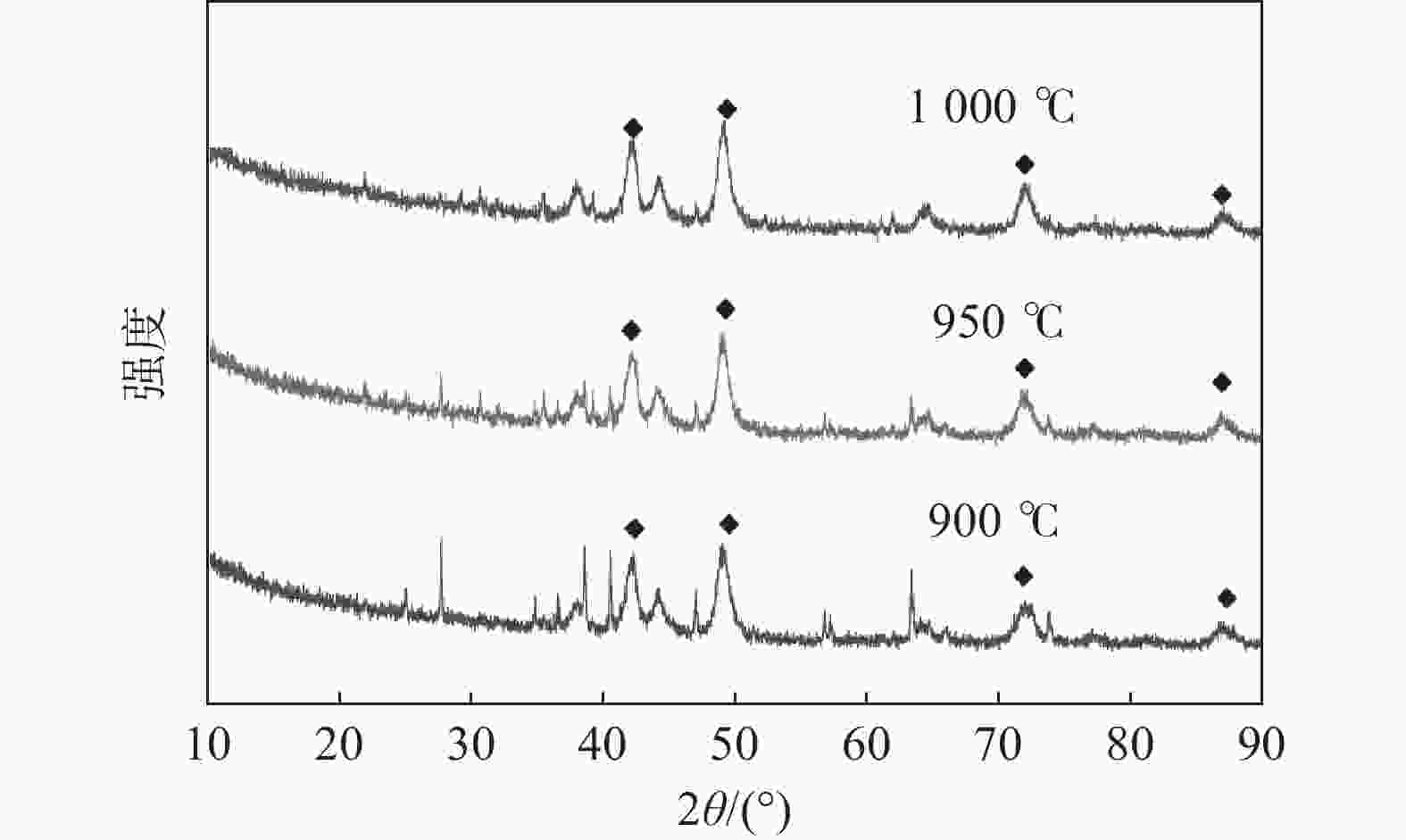
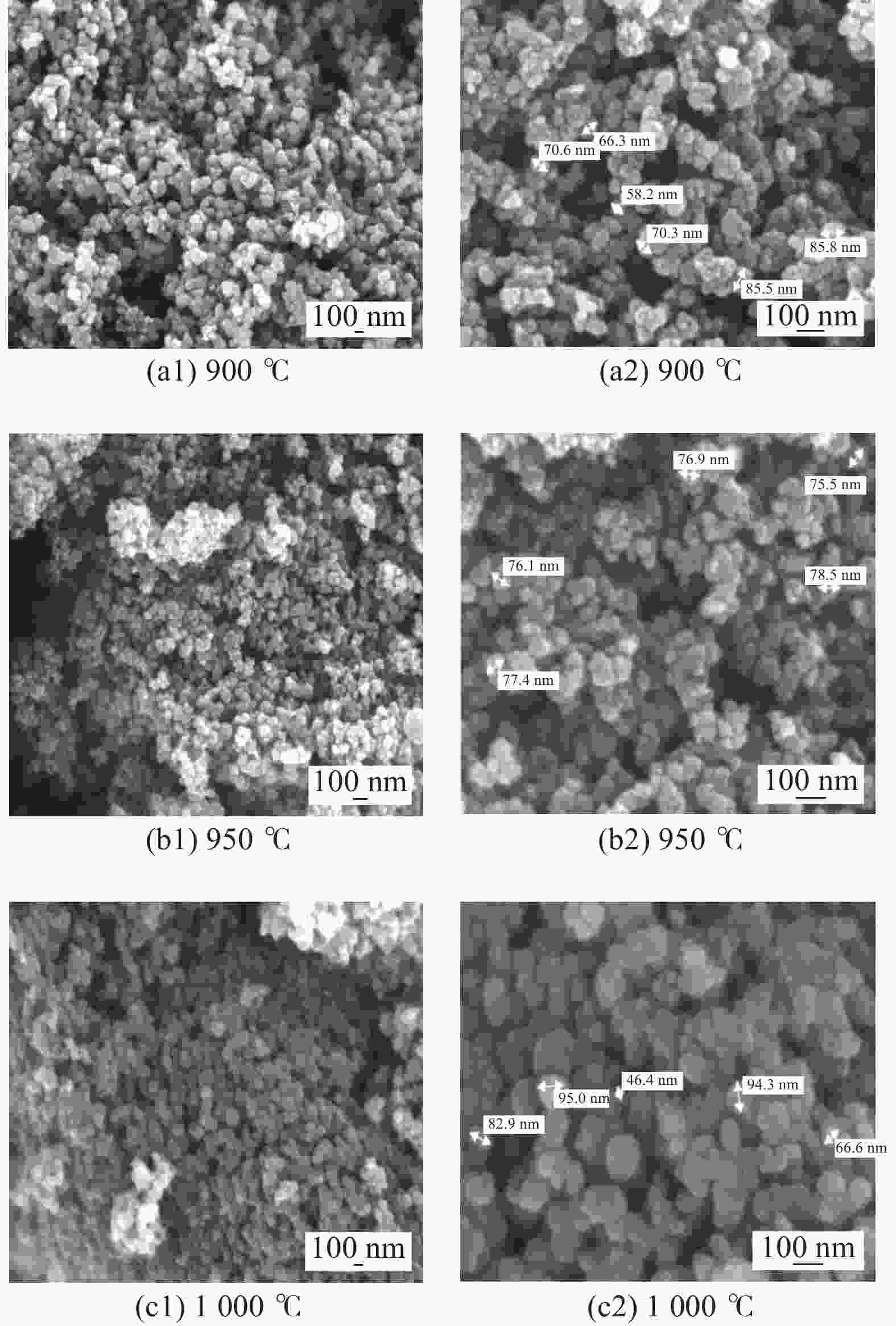
| [1] |
Liu Yang, Zhang Haodong, Yuan Suiyan, et al. Study on the mechanical properties of titanium carbide silicon ceramic materials[J]. Modern Salt Chemical Industry, 2021,(5):74−75. (刘洋, 张浩东, 原遂严, 等. 碳化钛硅陶瓷材料力学性能研究[J]. 现代盐化工, 2021,(5):74−75.Liu Yang, Zhang Haodong, Yuan Suiyan, et al. Study on the mechanical properties of titanium carbide silicon ceramic materials[J]. Modern Salt Chemical Industry, 2021, (5): 74-75
|
| [2] |
Liu Ping'an, Zeng Lingke, Shui Anze, et al. Research progress in the preparation and application of ultrafine titanium carbide powder[J]. Weapon Materials Science and Engineering, 2006,29(5):82−85. (刘平安, 曾令可, 税安泽, 等. 超细碳化钛粉体的制备及应用研究进展[J]. 兵器材料科学与工程, 2006,29(5):82−85.Liu Ping'an, Zeng Lingke, Shui Anze, et al. Research progress in the preparation and application of ultrafine titanium carbide powder[J]. Weapon Materials Science and Engineering, 2006, 29 (5): 82-85
|
| [3] |
Xu Benping. Current status and latest progress in chemical phase analysis of vanadium and titanium in vanadium and titanium materials[J]. China Inorganic Analytical Chemistry, 2014,4(2):18−23. (徐本平. 钒钛物料中钒钛化学物相分析现状及最新进展[J]. 中国无机分析化学, 2014,4(2):18−23.Xu Benping. Current status and latest progress in chemical phase analysis of vanadium and titanium in vanadium and titanium materials[J]. China Inorganic Analytical Chemistry, 2014, 4 (2): 18-23
|
| [4] |
Bandar Almangour, Min-seok Baek, Dariusz Grzesiak, et al. Strengthening of stainless steel by titanium carbide addition and grain refinement during selective laser melting [J]. Materials Science & Engineering A , 2017: S0921509317315.
|
| [5] |
Kattamis T Z, Suganuma T. Solidification processing and tribological behavior of particulate TiC-ferrous matrix composites[J]. Materials Science and Engineering A, 1990,128(2):241−252. doi: 10.1016/0921-5093(90)90232-R
|
| [6] |
Sen Wei, Xu Baoqiang, Yang Bin, et al. Preparation of titanium carbide powder by vacuum carbothermal reduction method[J]. Chinese Journal of Non Ferrous Metals:English Edition, 2011,21(1):185−190. (森维, 徐宝强, 杨斌, 等. 真空碳热还原法制备碳化钛粉末[J]. 中国有色金属学报:英文版, 2011,21(1):185−190.Sen Wei, Xu Baoqiang, Yang Bin, et al. Preparation of titanium carbide powder by vacuum carbothermal reduction method[J]. Chinese Journal of Non Ferrous Metals: English Edition, 2011, 21 (1): 185-190
|
| [7] |
Wang Zhi, Yuan Zhangfu, Guo Zhancheng. Exploration of a new process for direct preparation of titanium alloy by molten salt electrolysis[J]. Non-ferrous Metals, 2003,55(4):32-43. (王志, 袁章福, 郭占成. 熔盐电解法直接制备钛合金新工艺探讨[J]. 有色金属, 2003,55(4):32-43.Wang Zhi, Yuan Zhangfu, Guo Zhancheng. Exploration of a new process for direct preparation of titanium alloy by molten salt electrolysis[J]. Non-ferrous Metals, 2003, 55 (4): 4
|
| [8] |
Hao Xian, Liang Feng, Li Hongxia, et al. Progress in the preparation of nano titanium carbide and its application in energy storage[J]. Material Introduction, 2021,35(S01):11-18. (郝娴, 梁峰, 李红霞, 等. 纳米碳化钛的制备及在储能领域的应用研究进展[J]. 材料导报, 2021,35(S01):11-18.Hao Xian, Liang Feng, Li Hongxia, et al. Progress in the preparation of nano titanium carbide and its application in energy storage[J]. Material Introduction, 2021, 35 (S01): 8
|
| [9] |
Wang Jinshu, Xing Pengfei, Li Lili, et al. Preparation and characterization of N doped nano TiO2 by mechanochemical method[J]. Journal of Beijing University of Technology, 2006,32(7):633−637. (王金淑, 邢朋飞, 李莉莉, 等. 机械化学法N掺杂纳米TiO2的制备与表征[J]. 北京工业大学学报, 2006,32(7):633−637.Wang Jinshu, Xing Pengfei, Li Lili, et al. Preparation and characterization of N doped nano TiO2 by mechanochemical method[J]. Journal of Beijing University of Technology, 2006, 32 (7): 633-637
|
| [10] |
Luo Xuming, Chen Yiming, Mu Yufeng. Self propagating high-temperature synthesis of non stoichiometric titanium carbide based cermets[J]. Material Development and Application, 1995,(5):20−23. (罗序明, 陈一鸣, 母育锋. 自蔓延高温合成非化学计量碳化钛基金属陶瓷[J]. 材料开发与应用, 1995,(5):20−23.Luo Xuming, Chen Yiming, Mu Yufeng. Self propagating high-temperature synthesis of non stoichiometric titanium carbide based cermets[J]. Material Development and Application, 1995, (5): 20-23
|
| [11] |
Lv Yanan, Chen Dong. Study on atomic diffusion behavior during the formation of titanium carbide particles[J]. Iron Steel Vanadium Titanium, 2021,42(3):143−147. (吕亚男, 陈栋. 碳化钛颗粒形成过程中原子扩散行为研究[J]. 钢铁钒钛, 2021,42(3):143−147.Lv Yanan, Chen Dong. Study on atomic diffusion behavior during the formation of titanium carbide particles[J]. Iron Steel Vanadium Titanium, 2021, 42 (3): 143-147
|
| [12] |
Zhou Yanli. Research on the salt separation process of sodium chloride and potassium chloride wastewater[J]. Hebei Chemical Industry, 2021,44(1):136−138. (周艳丽. 氯化钠氯化钾废水分盐工艺研究[J]. 河北化工, 2021,44(1):136−138.Zhou Yanli. Research on the salt separation process of sodium chloride and potassium chloride wastewater[J]. Hebei Chemical Industry, 2021, 44 (1): 136-138
|




 下载:
下载: