Study on leaching behavior of vanadium in acid leaching process of calcium and manganese vanadate system
-
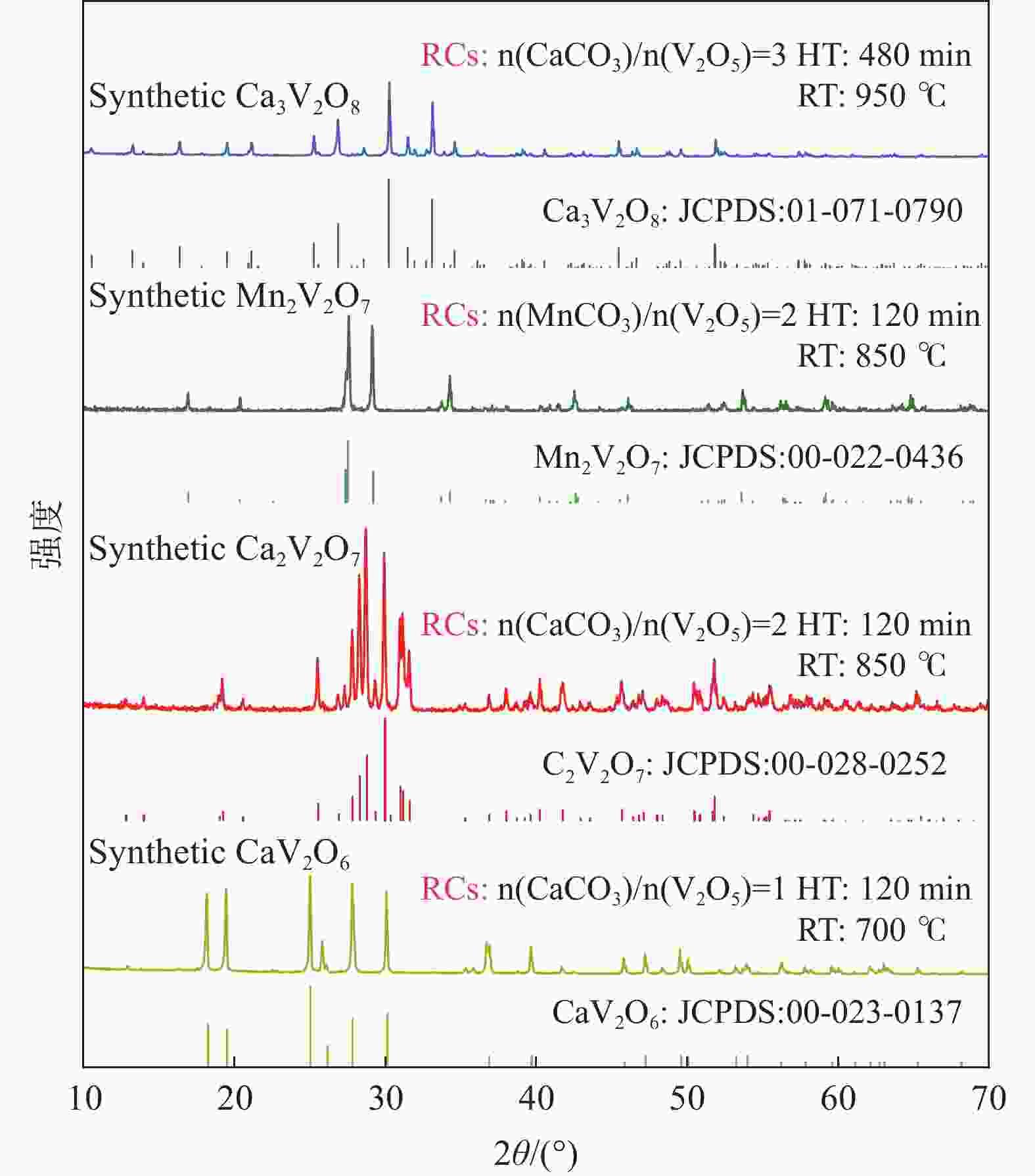
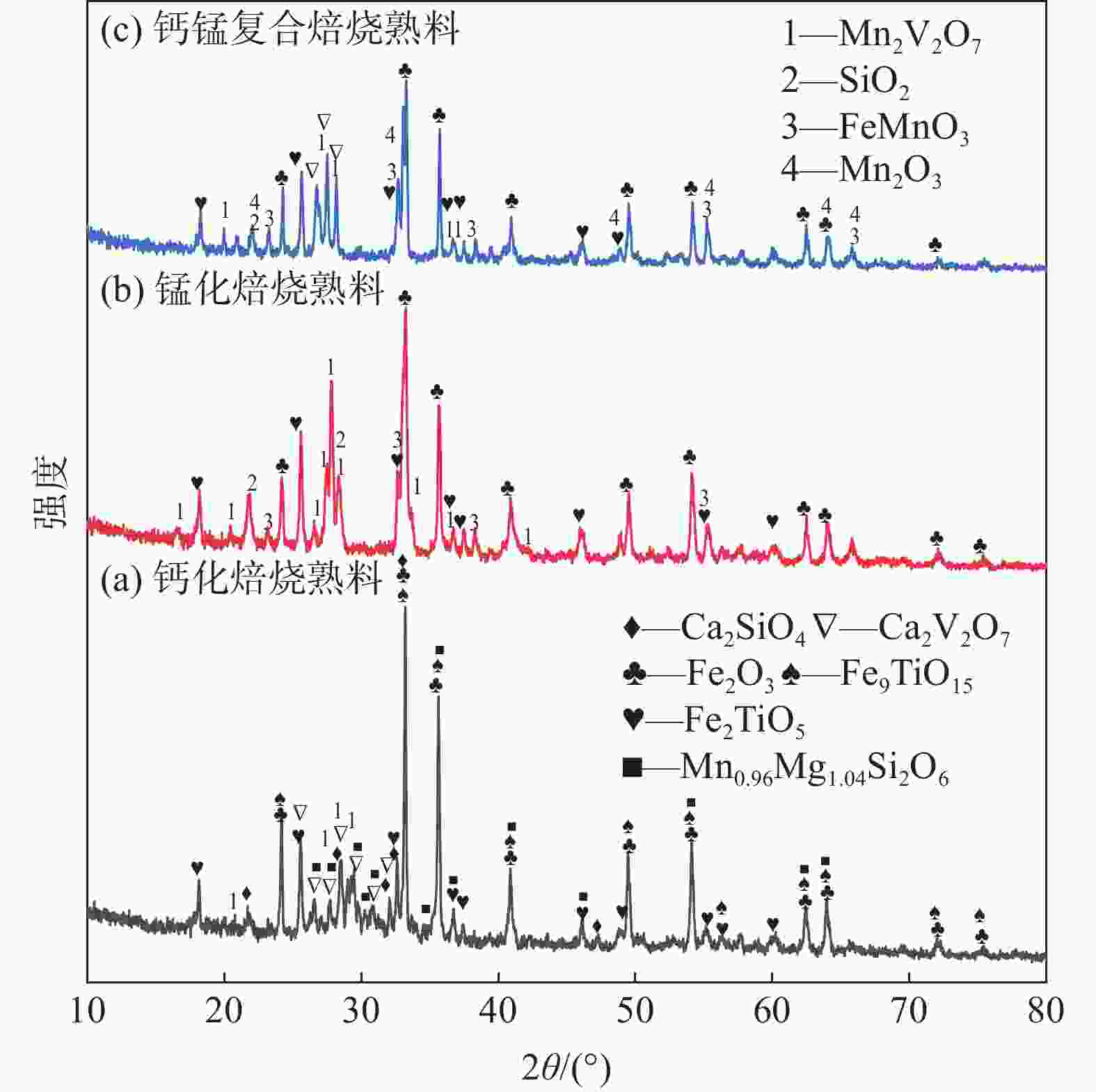
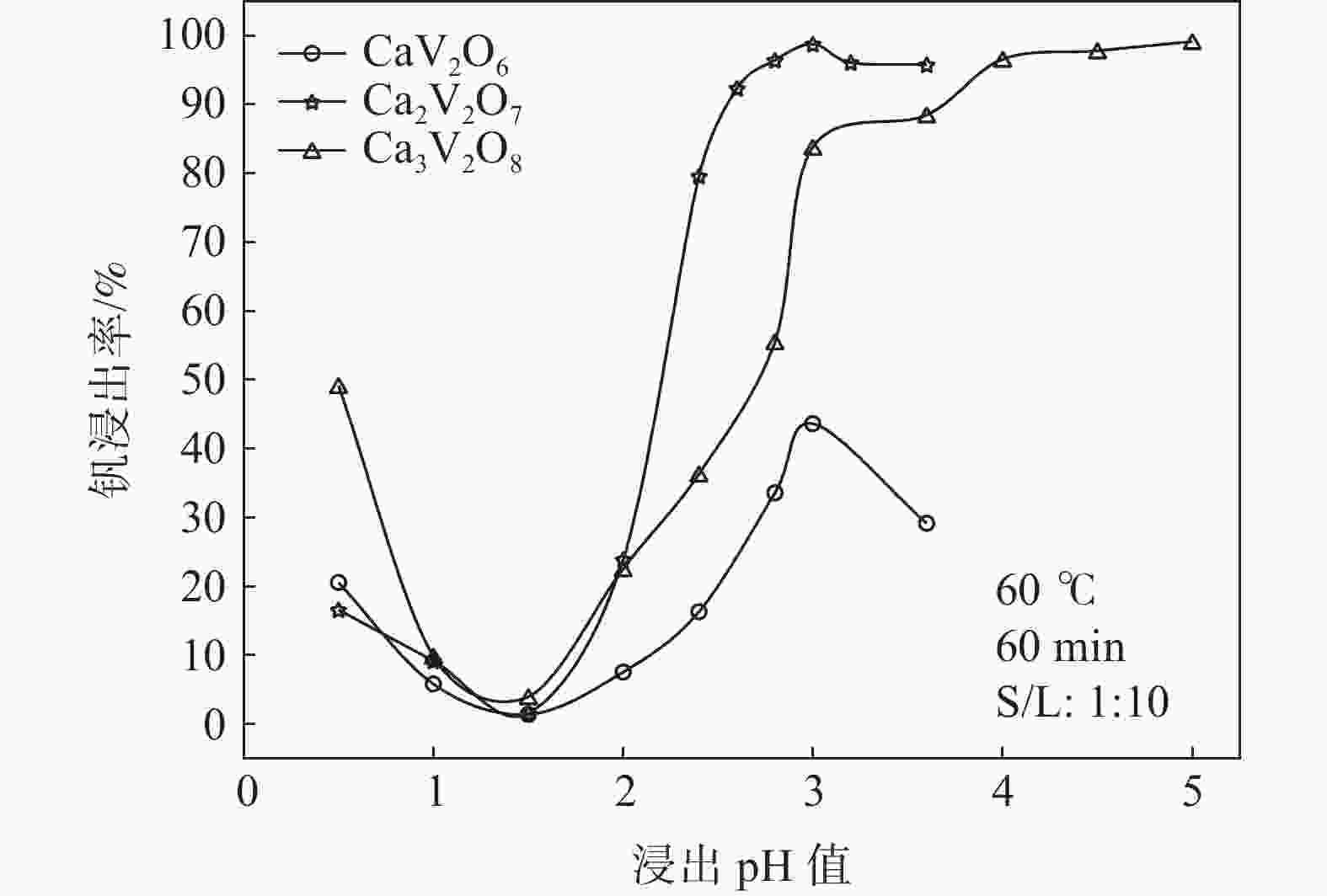
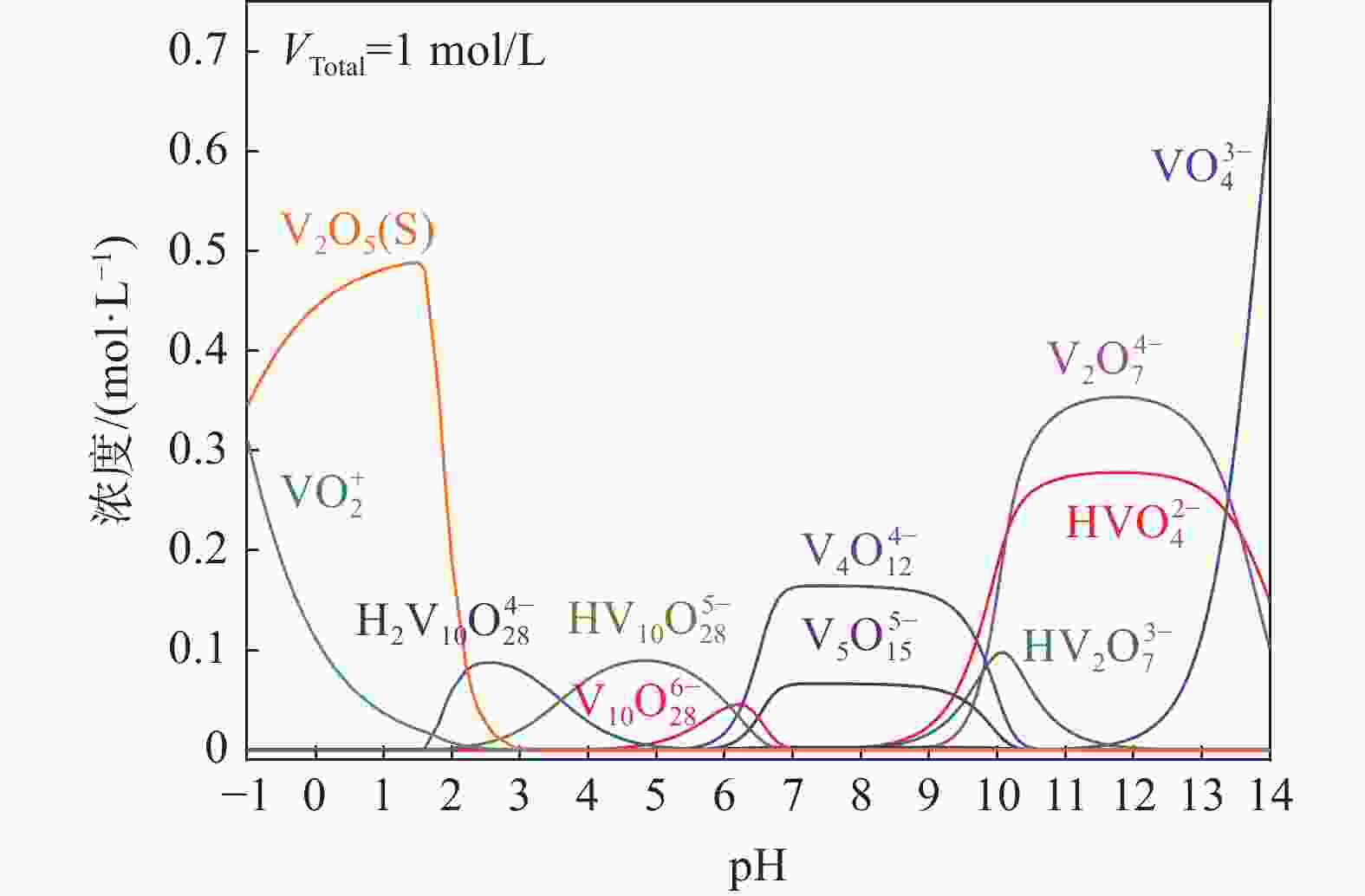
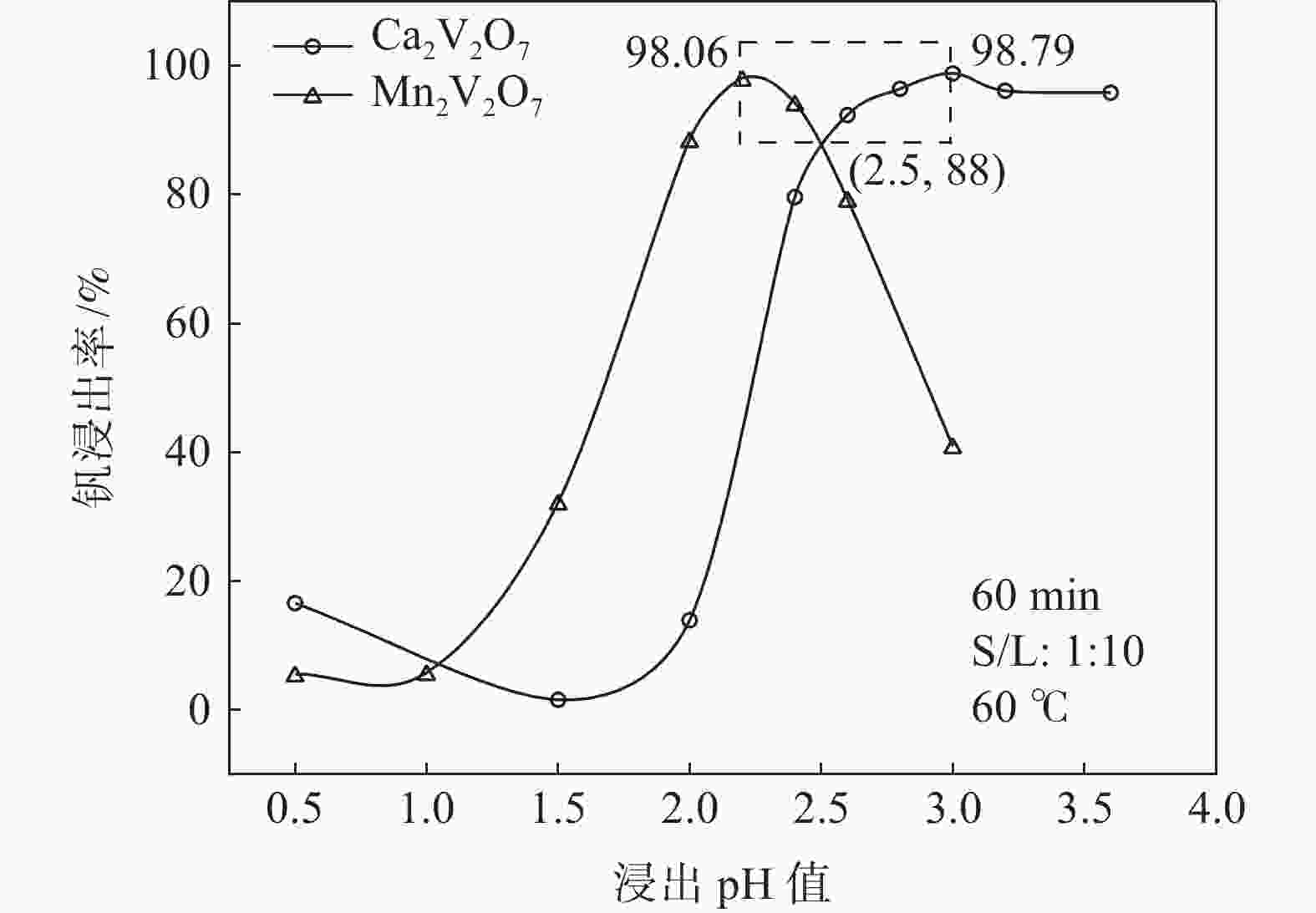
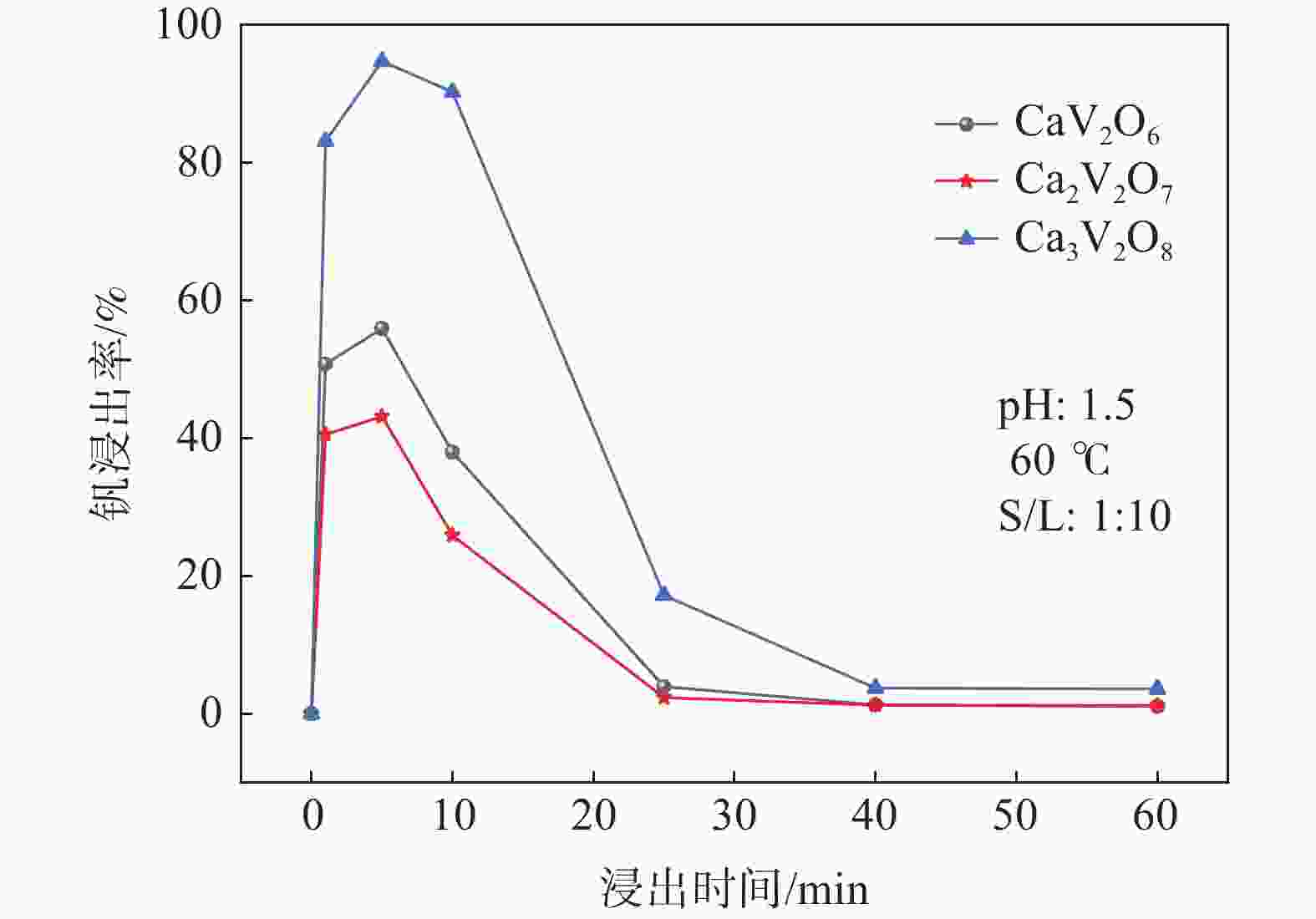
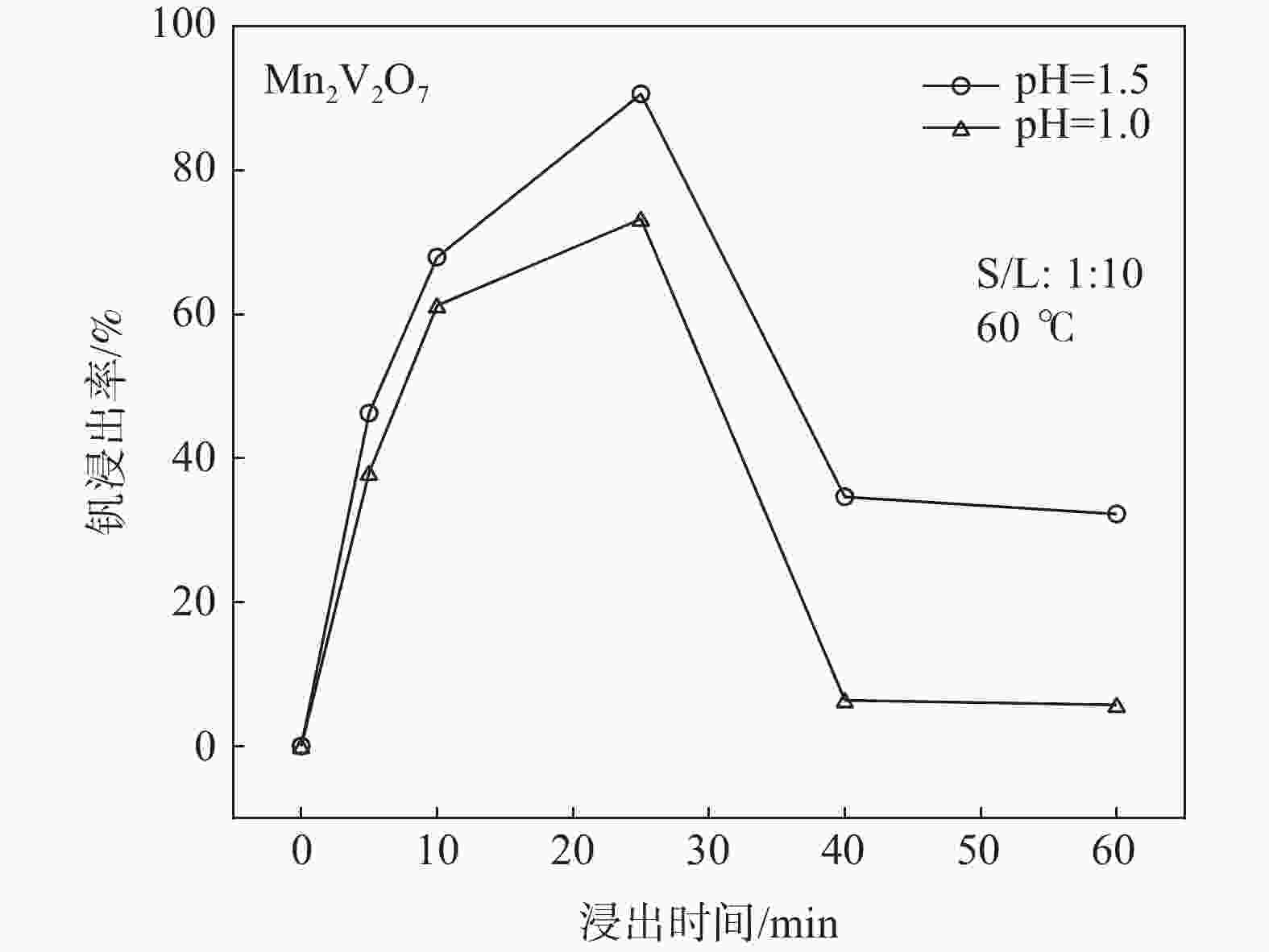
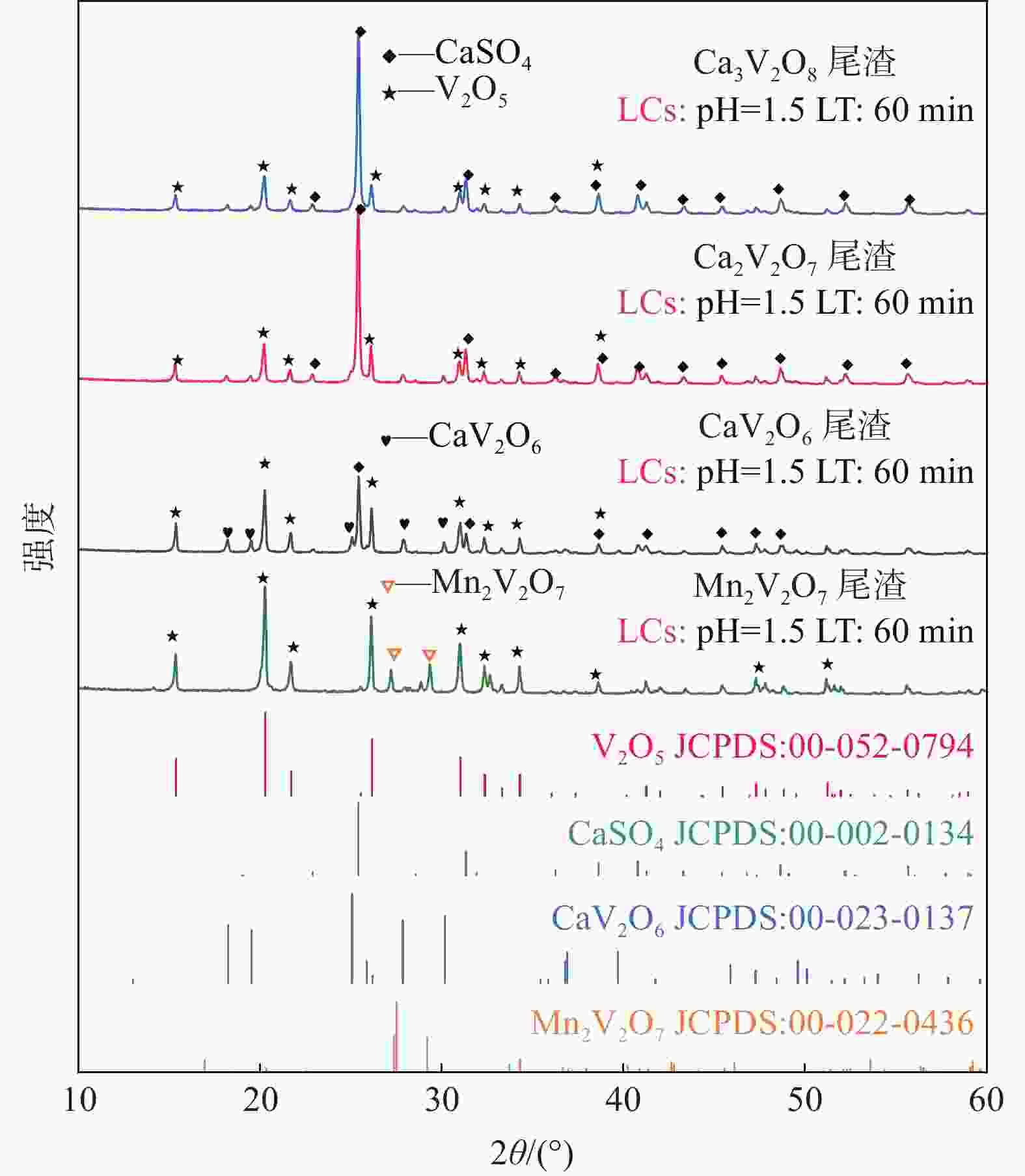
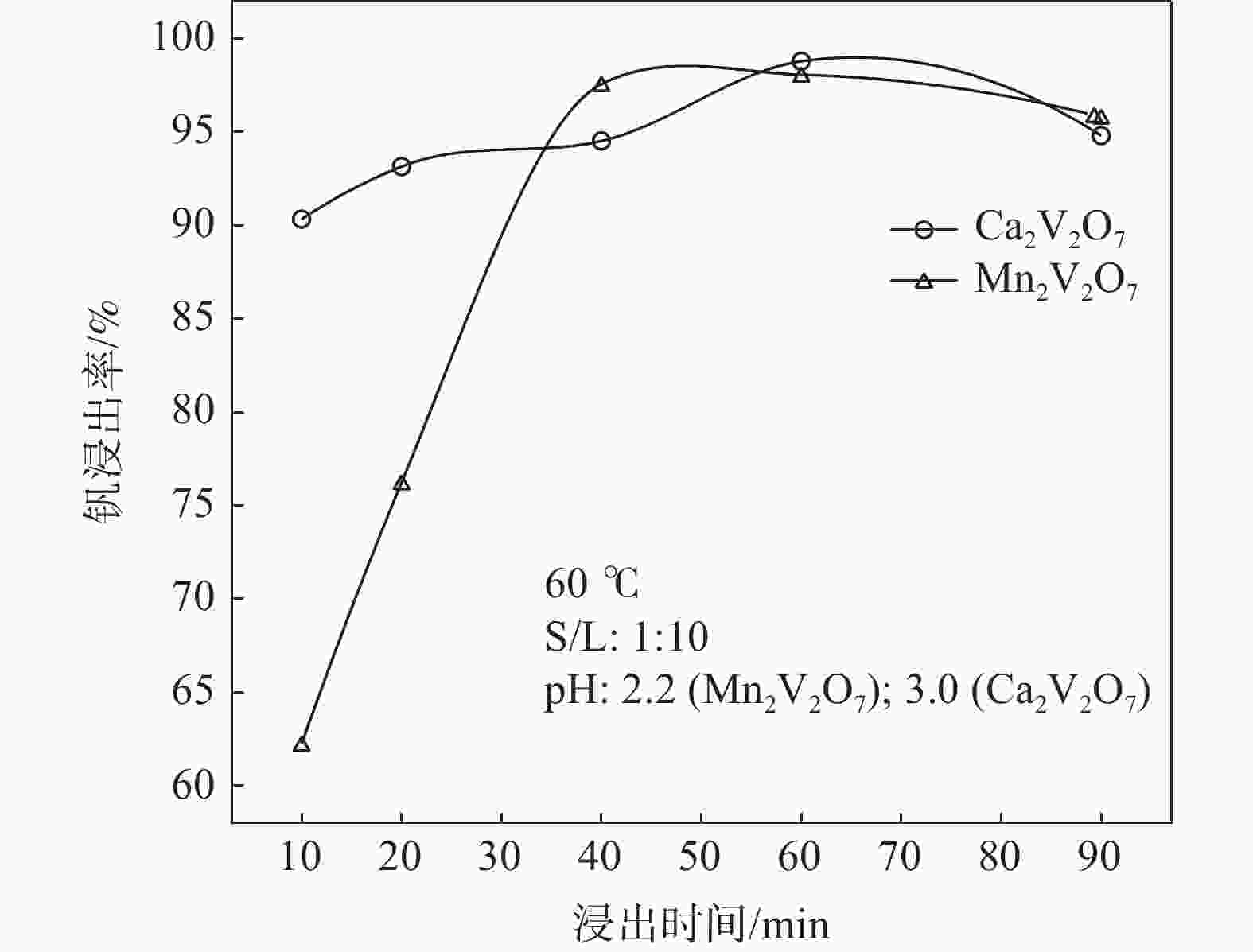
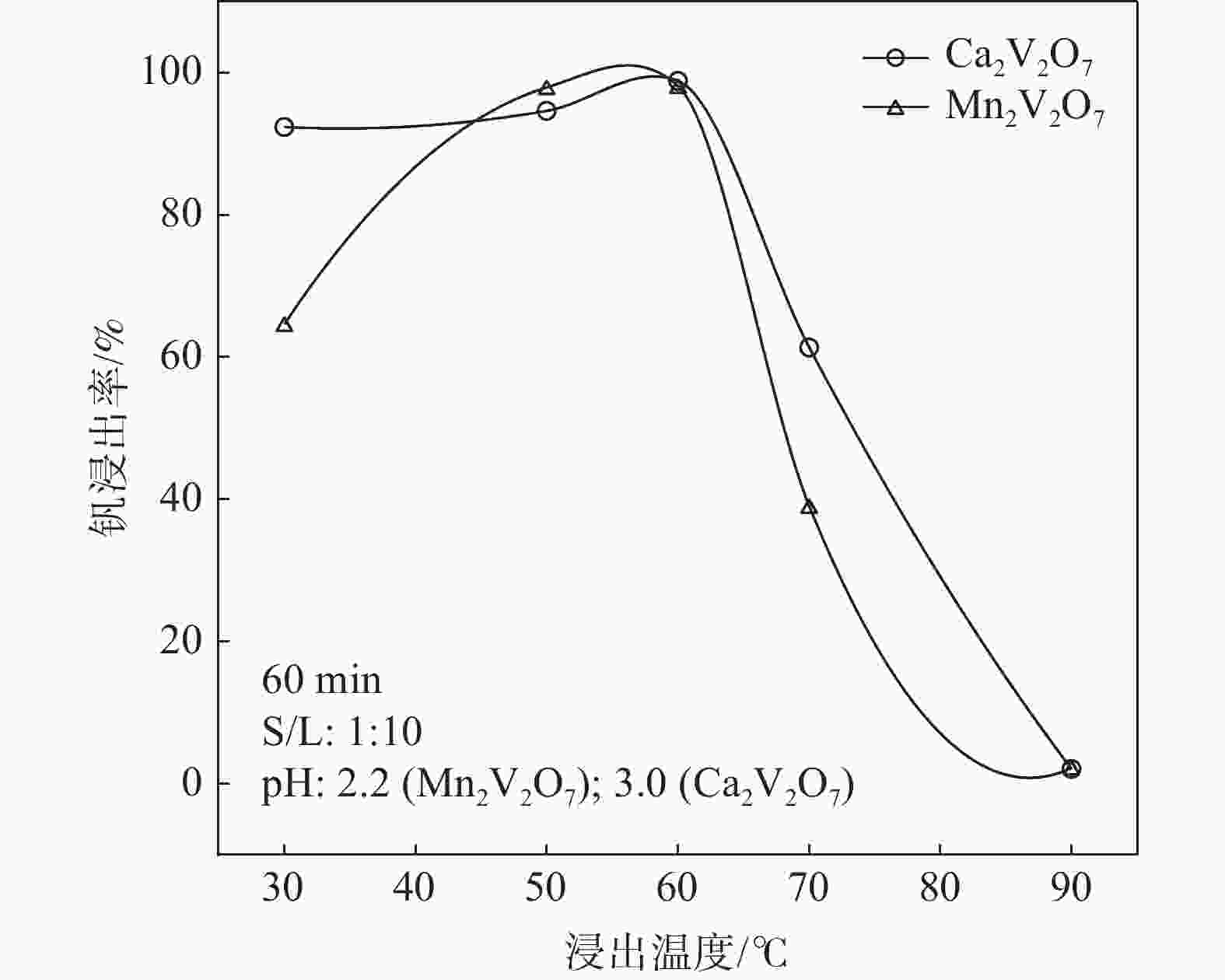
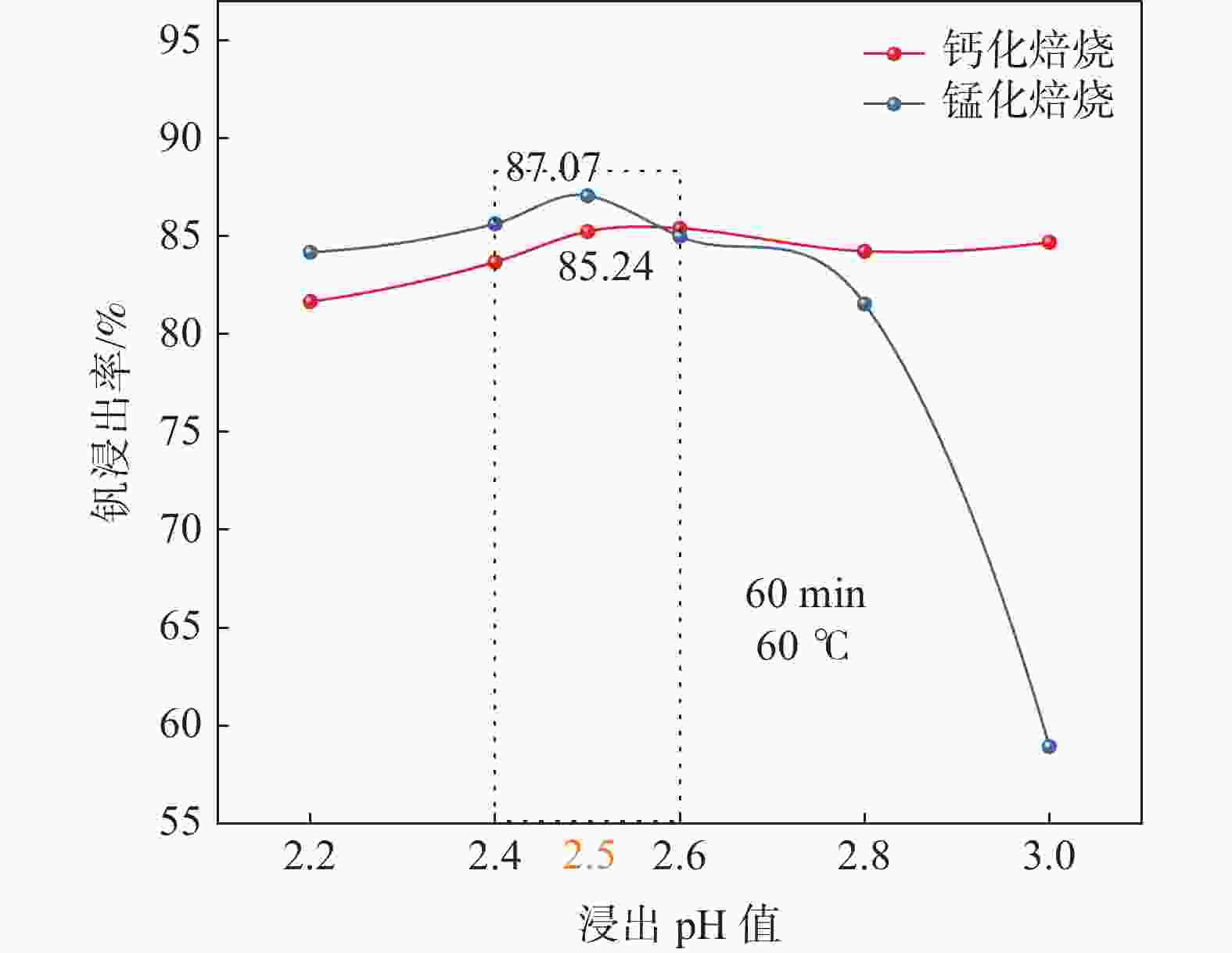
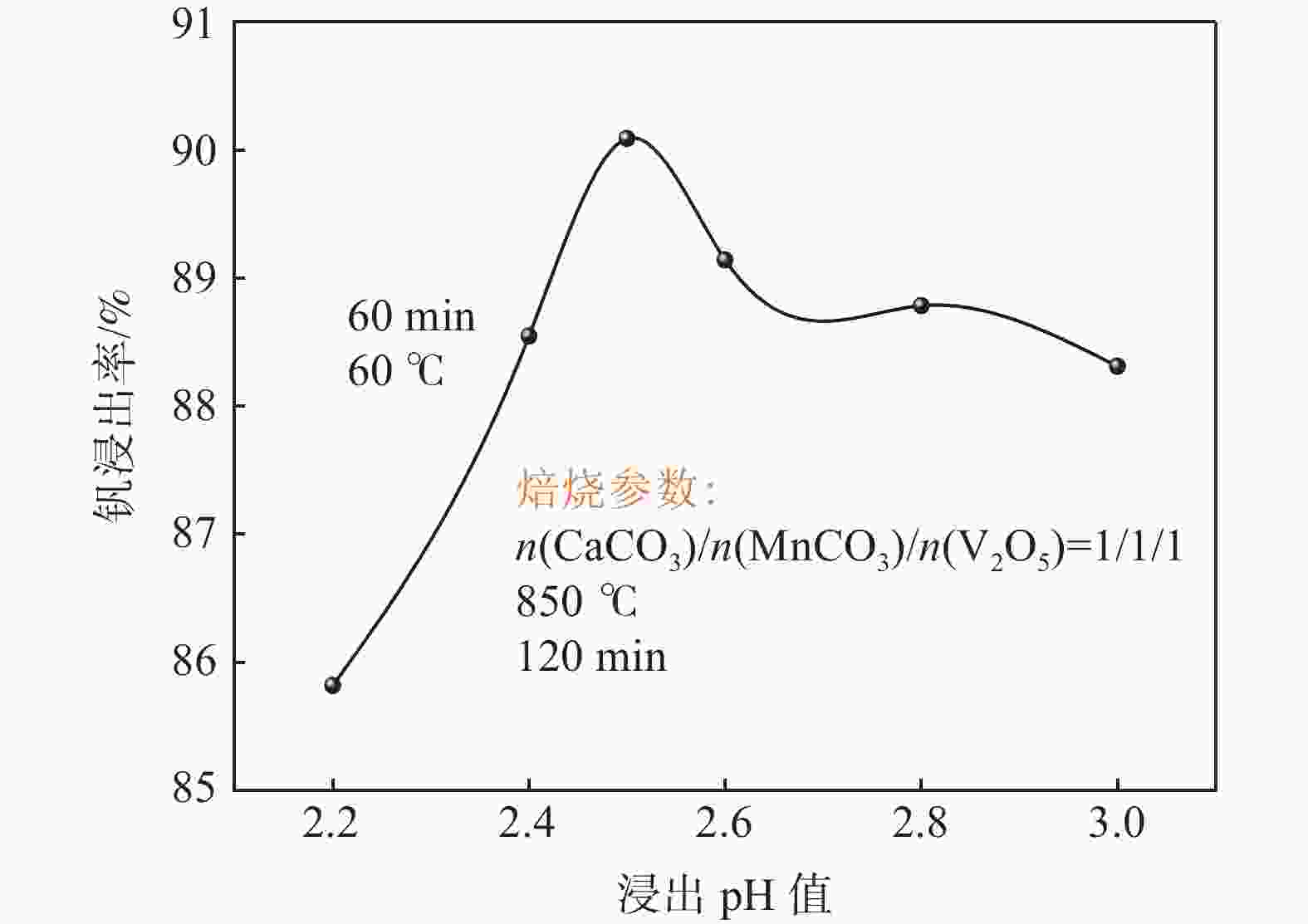
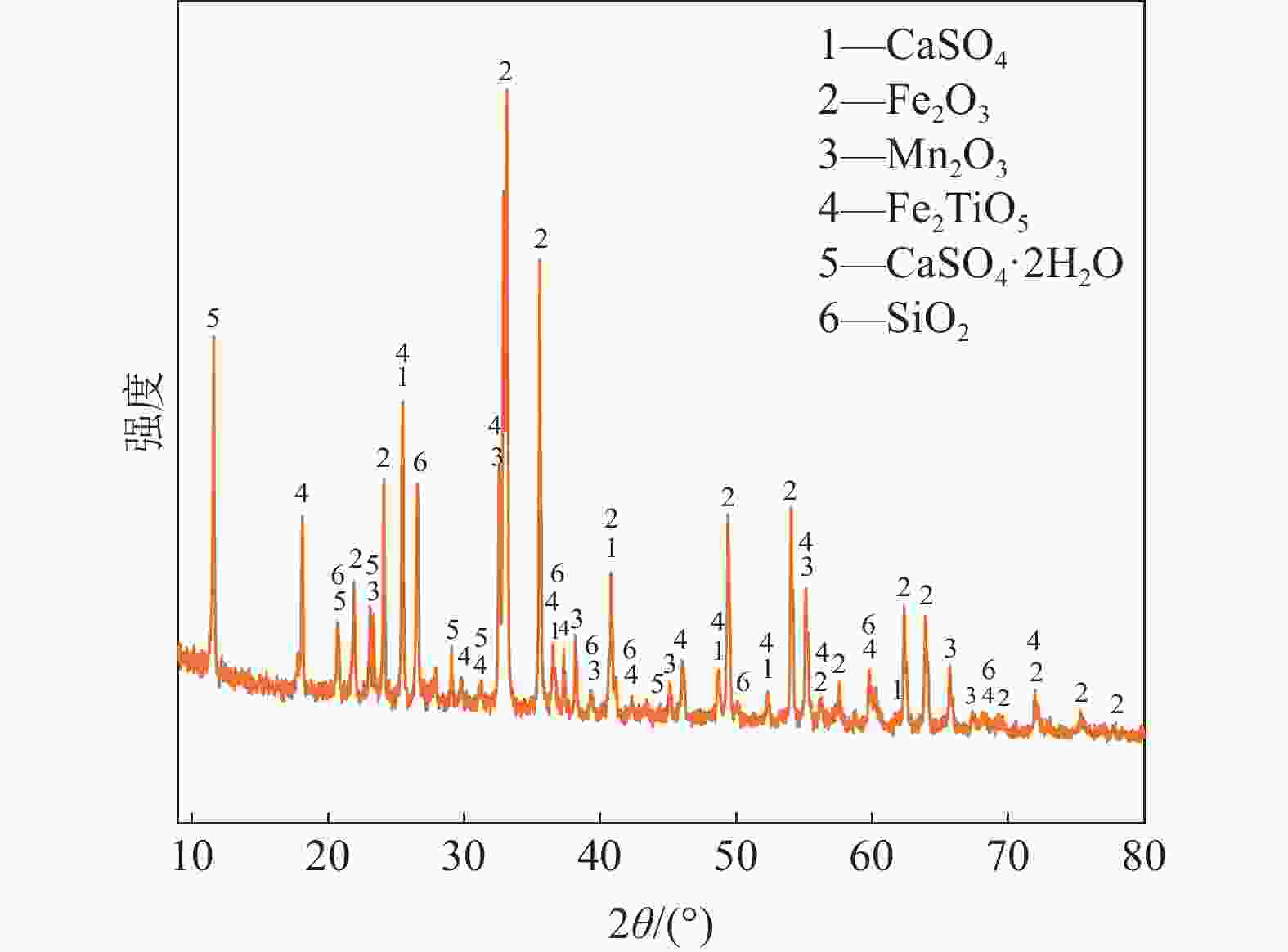
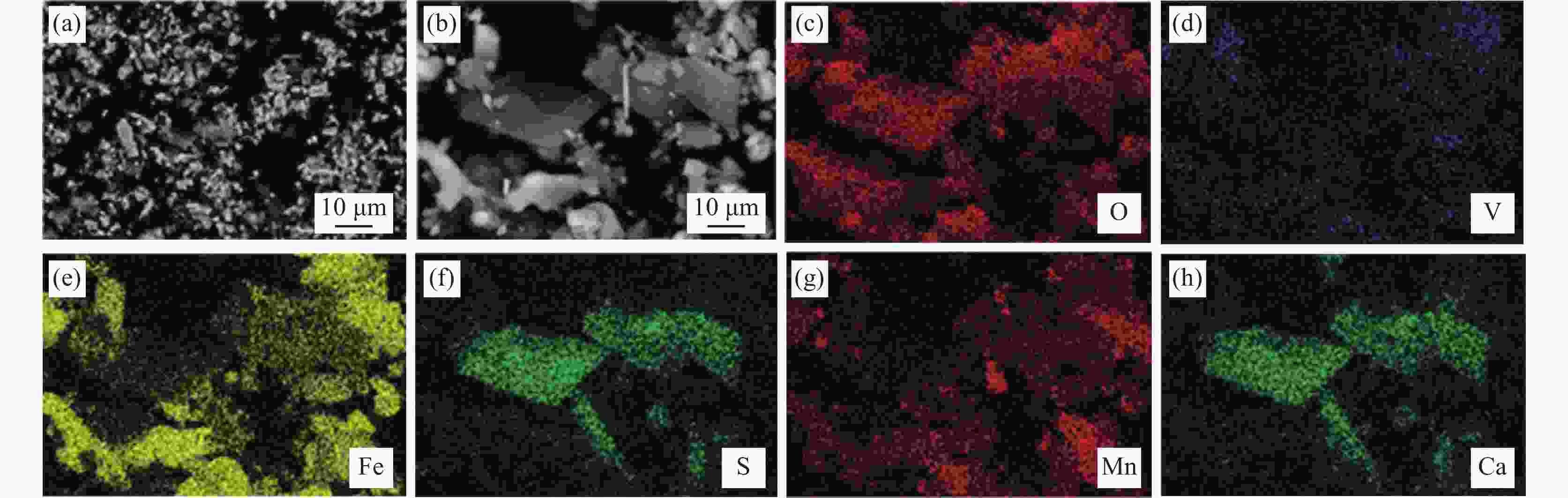
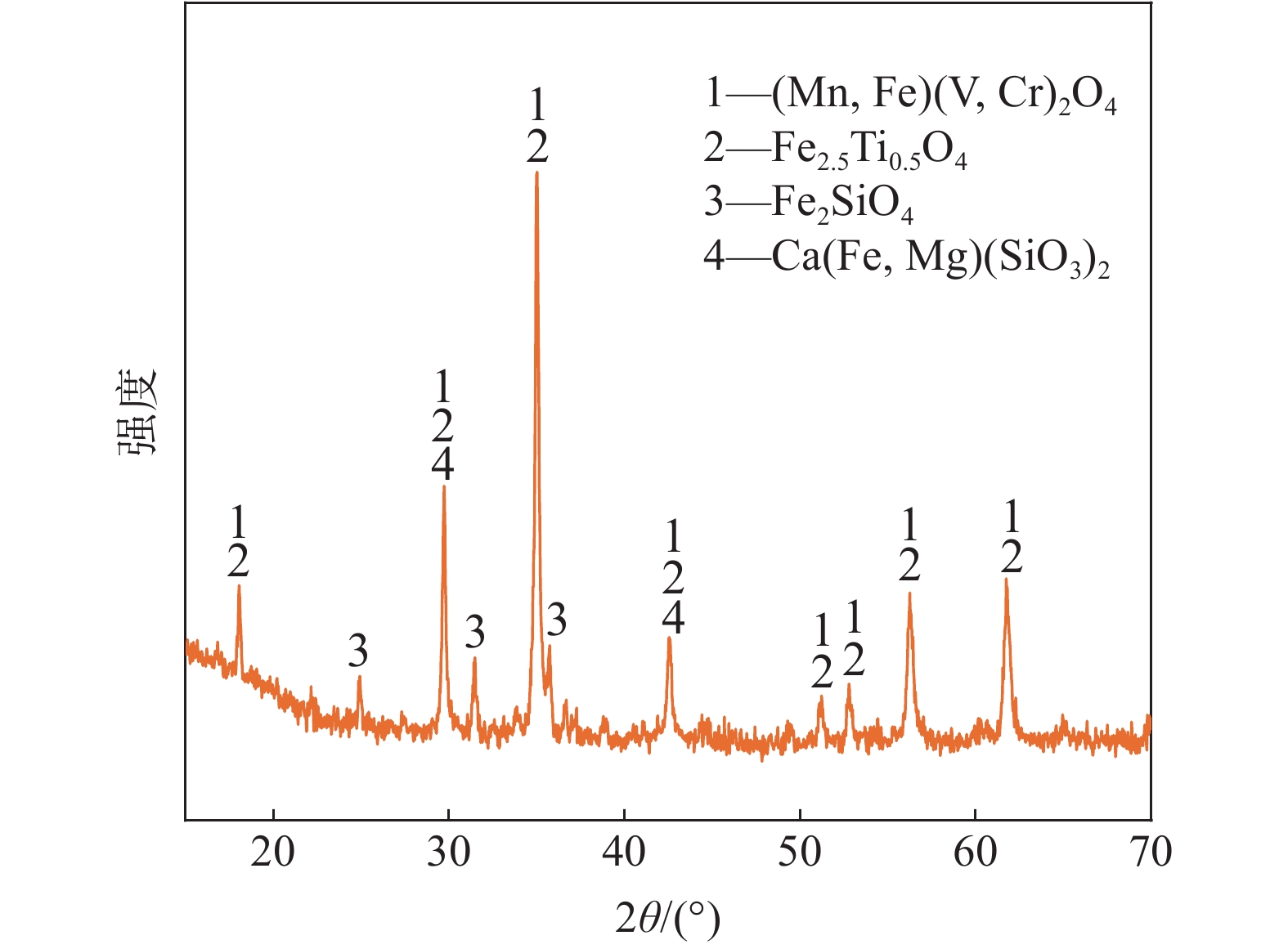
摘要: 钒酸钙和钒酸锰是钒渣钙化焙烧过程中重要的焙烧产物,其在硫酸中的溶解浸出行为对提钒过程钒收率有重要影响。采用单因素试验递进式研究了浸出pH值、时间及温度对合成钒酸钙(锰)以及钒渣钙化(锰化)、钙锰复合焙烧熟料中钒组元浸出行为的影响。结果表明,合成CaV2O6、Ca2V2O7、Ca3V2O8及Mn2V2O7在pH值分别为3.0、3.0、5.0、2.2的硫酸溶液中于60 ℃下浸出60 min时,钒的浸出率达到最大值,分别为43.62%、98.79%、97.98%、98.06%。pH值为1.0~2.0时,四种钒酸盐钒的浸出率均出现一个低谷,这是由钒的水解引起的。钒渣常规钙化和锰化焙烧过程中主要生成Ca2V2O7和Mn2V2O7两种钒酸盐,浸出pH值为2.5时,钒的浸出率分别为85.24%、87.07%;钒渣钙锰复合焙烧过程中生成的焦钒酸钙、焦钒酸锰共生复盐,熟料于pH值为2.5的硫酸溶液中浸出后钒的浸出率最大为90.09%,较常规钙化、锰化体系钒的浸出率高约四个百分点,表明钙、锰协同提钒与单一添加剂提钒相比有一定的强化作用。Abstract: Calcium vanadate and manganese vanadate are important roasting products in the calcification roasting process of vanadium slag, and their dissolution and leaching behavior in sulfuric acid has an important effect on the vanadium yield in the vanadium extraction process. In this paper, the effects of leaching pH, time and temperature on the leaching behavior of vanadium components in the synthesis of calcium vanadate (or manganese vanadate), vanadium slag calcification (or reaction with manganese) and calcium-manganese composite roasting clinker were studied by single factor test step-by-step method. The results showed that the maximum leaching rate of vanadium was 43.62%, 98.79%, 97.98% and 98.06% when the synthetic CaV2O6, Ca2V2O7, Ca3V2O8 and Mn2V2O7 were leached at 60℃ for 60 min at pH 3.0, 3.0, 5.0 and 2.2, respectively. When the pH value is 1.0~2.0, the leaching rate of the four vanadates shows a low peak, which is caused by the hydrolysis of vanadium. Ca2V2O7 and Mn2V2O7 vanadate were mainly formed during the conventional calcification and manganese roasting of vanadium slag, and the leaching rates of vanadium were 85.24% and 87.07%, respectively, when the leaching pH value was 2.5. The leaching rate of vanadium after clinker leaching in sulfuric acid solution with pH value of 2.5 was 90.09%, which was about four percentage points higher than that of vanadium in conventional calcification and manganese systems, indicating that the synergistic extraction of vanadium from calcium and manganese had a certain strengthening effect on vanadium extraction with a single additive.
-
Key words:
- vanadium slag /
- calcification roasting /
- calcium vanadate /
- manganese vanadate /
- leaching behavior
-
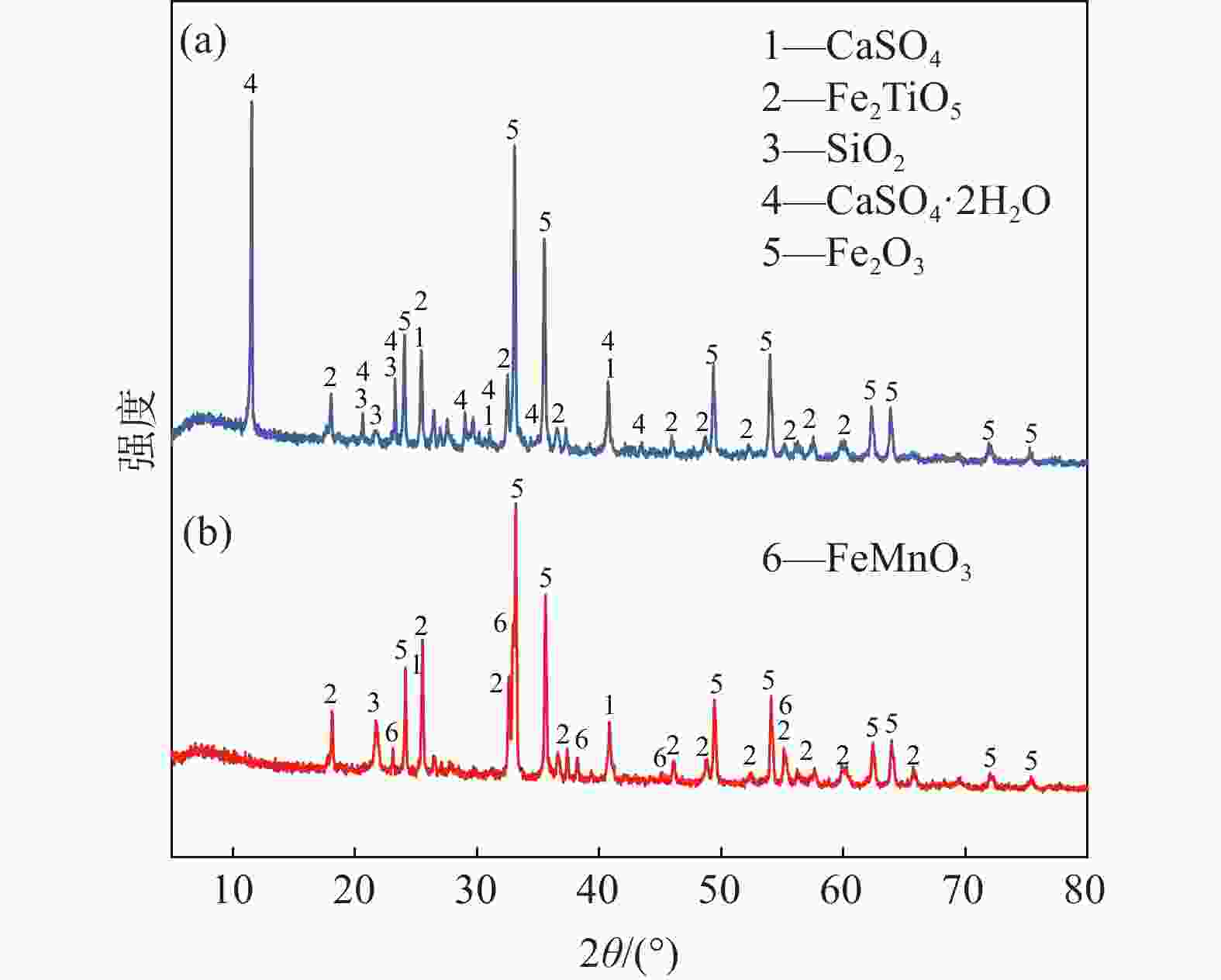
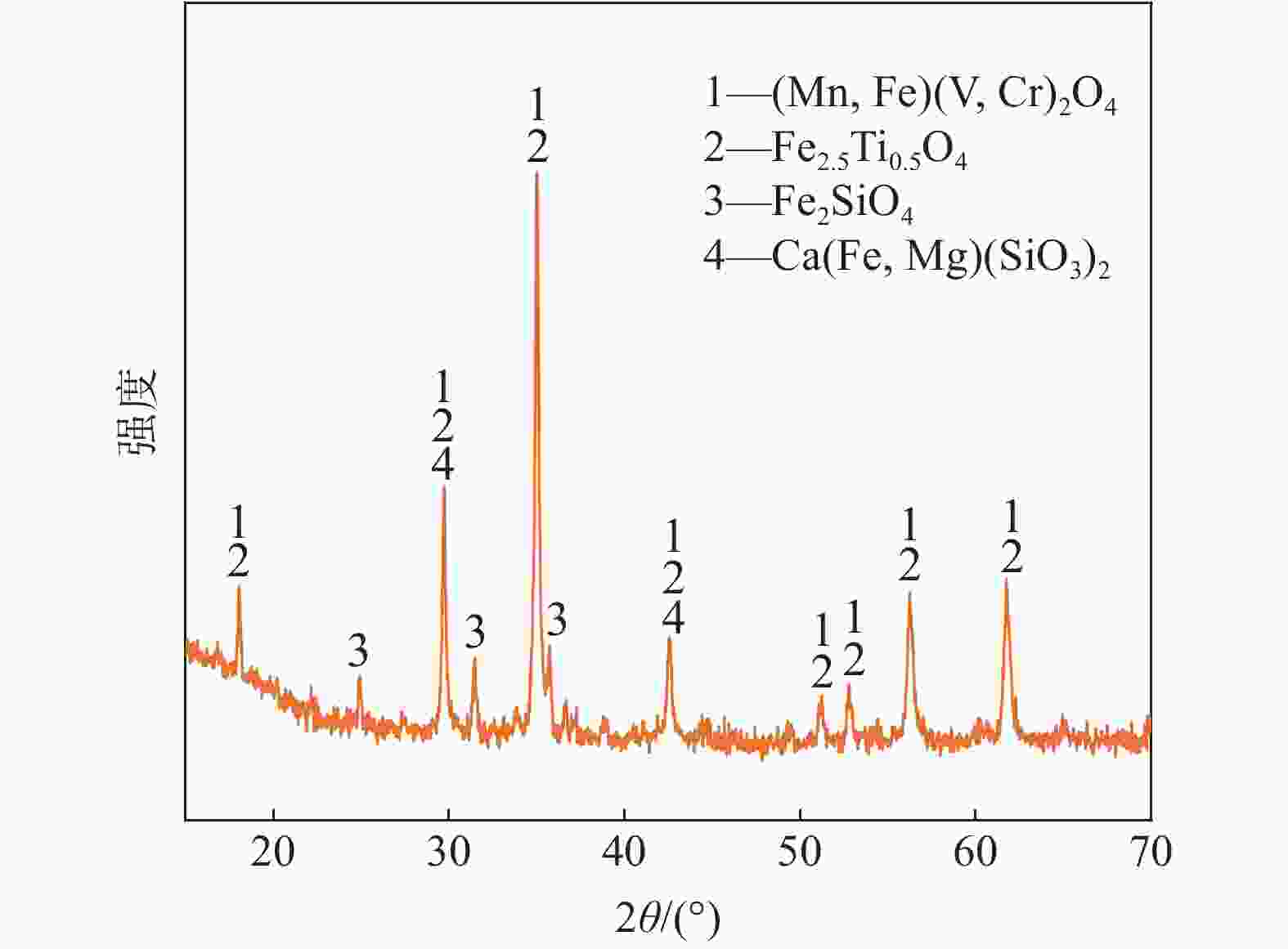
表 1 钒渣主要化学成分
Table 1. Main chemical constituents of vanadium slag
% FeO V2O3 MnO TiO2 CaO Cr2O3 SiO2 Total 38.78 14.81 8.75 11.44 2.47 2.19 14.30 92.74 -
[1] Yang Shouzhi. Vanadium metallurgy [M]. Beijing: Metallurgical Industry Press, 2010. (杨守志. 钒冶金[M]. 北京: 冶金工业出版社, 2010.Yang Shouzhi. Vanadium metallurgy [M]. Beijing: Metallurgical Industry Press, 2010. [2] Qi Mingjian. Present situation and prospect of extracting vanadium from stone coal[J]. Hydrometallurgy, 1999(4):1−10. (漆明鉴. 从石煤中提钒现状及前景[J]. 湿法冶金, 1999(4):1−10.Qi Mingjian. Present situation and prospect of extracting vanadium from stone coal[J]. Hydrometallurgy, 1999(4): 1−10. [3] Liao Shiming , Bai Tanlun. Foreign vanadium metallurgy[M]. Beijing: Metallurgical Industry Press, 1985. (廖世明, 柏谈论. 国外钒冶金[M]. 北京: 冶金工业出版社, 1985.Liao Shiming , Bai Tanlun. Foreign vanadium metallurgy[M]. Beijing: Metallurgical Industry Press, 1985. [4] Wang Shuai, Guo Yufeng, Zheng Fuqiang, et al. Behavior of vanadium during reduction and smelting of vanadium titanomagnetite metallized pellets[J]. Transactions of Nonferrous Metals Society of China, 2020,30:1687−1696. (王帅, 郭玉峰, 郑富强, 等. 钒钛磁铁矿金属化球团还原冶炼过程中钒的行为[J]. 有色金属学报, 2020,30:1687−1696.Wang Shuai, Guo Yufeng, Zheng Fuqiang, et al. Behavior of vanadium during reduction and smelting of vanadium titanomagnetite metallized pellets[J]. Transactions of Nonferrous Metals Society of China, 2020, 30: 1687−1696. [5] Chen Donghui. 2021 annual evaluation of vanadium industry[J]. Hebei Metallurgy, 2022,324(12):19−30. (陈东辉. 钒产业2021年年度评价[J]. 河北冶金, 2022,324(12):19−30.Chen Donghui. 2021 annual evaluation of vanadium industry[J]. Hebei Metallurgy, 2022, 324(12): 19−30. [6] Zhang X, Xie B, Diao J, et al. Nucleation and growth kinetics of spinel crystals in vanadium slag[J]. Ironmaking & Steelmaking, 2013,39:147−154. [7] Deng Rongrui, Xiao Hao, Xie Zhaoming, et al. A novel method for extracting vanadium by low temperature sodium roasting from converter vanadium slag[J]. Chinese Journal Chemical Engineering, 2020,28(8):2208−2213. doi: 10.1016/j.cjche.2020.03.038 [8] Liu Biao, Du Hao, Wang Shaona, et al. A novel method to extract vanadium and chromium from vanadium slag using molten NaOH-NaNO3 binary system[J]. AIChE Journal. 2023, 59 (2): 541–552. [9] Li Kunlin, Ren Shaolong, Cheng Feng. Experimental study on vanadium extraction from high-calcium and low-grade vanadium slag[J]. Iron Steel Vanadium Titanium, 2014,35(1):16−20. (李坤林, 任少龙, 成丰. 高钙低品位钒渣提钒试验研究[J]. 钢铁钒钛, 2014,35(1):16−20.Li Kunlin, Ren Shaolong, Cheng Feng. Experimental study on vanadium extraction from high-calcium and low-grade vanadium slag[J]. Iron Steel Vanadium Titanium, 2014, 35(1): 16−20. [10] Wu Enhui, Hou Jing, Li Jun. Experimental study on oxidative calcification roasting-acid leaching of vanadium from vanadium chromium residue[J]. Rare Metals and Cemented Carbid, 2017,45(6):8−13. (吴恩辉, 侯静, 李军. 钒铬渣氧化钙化焙烧-酸浸提钒实验研究[J]. 稀有金属与硬质合金, 2017,45(6):8−13.Wu Enhui, Hou Jing, Li Jun. Experimental study on oxidative calcification roasting-acid leaching of vanadium from vanadium chromium residue[J]. Rare Metals and Cemented Carbid, 2017, 45(6): 8−13. [11] Zhang Juhua, Zhang Wei, Xue Zhengliang. An environment-friendly process featuring calcified roasting and precipitation purification to prepare vanadium pentoxide from the converter vanadium slag[J]. Metals, 2018,9(1):21. doi: 10.3390/met9010021 [12] Zhang Juhua, Yan Zhefeng, Feng Jiaxin, et al. Effect of vanadium slag particle size on vanadium slag calcification roasting process of vanadium extraction[J]. Journal of Anhui University of Technology(Natural Science Edition), 2017, 34(4): 316-321, 326. ) (张菊花, 严哲锋, 冯嘉鑫, 等. 钒渣粒径对钒渣钙化焙烧提钒过程的影响[J]. 安徽工业大学学报(自然科学版), 2017, 34(4): 316−321, 326.Zhang Juhua, Yan Zhefeng, Feng Jiaxin, et al. Effect of vanadium slag particle size on vanadium slag calcification roasting process of vanadium extraction[J]. Journal of Anhui University of Technology(Natural Science Edition), 2017, 34(4): 316-321, 326. ) [13] Gao Huiyang, Jiang Tao, Zhou Mi. Effect of microwave irradiation and conventional calcification roasting with calcium hydroxide on the extraction of vanadium and chromium from high−chromium vanadium slag[J]. Mineral Processing, 2020,145:106056. [14] Cao Peng. Research on vanadium slag roasted with calcium salt[J]. Iron Steel Vanadium Titanium, 2012,13(1):30−34. (曹鹏. 钒渣钙化焙烧试验研究[J]. 钢铁钒钛, 2012,13(1):30−34.Cao Peng. Research on vanadium slag roasted with calcium salt[J]. Iron Steel Vanadium Titanium, 2012, 13(1): 30−34. [15] Jiang Tao, Wen Jing, Zhou Mi, et al. Phase evolutions, microstructure and reaction mechanism during calcification roasting of high chromium vanadium slag[J]. Joural of Alloy and Compounds: An Interdisciplinary Journal of Materials Science and Solidstate Chemistry and Physics, 2018,742:402−412. [16] Wen Jing, Jiang Tao, Sun Hongyan, et al. Investigation on separation principle of vanadium and chromium among Fe2VO4-CaO-FeCr2O4 system: Simplify and simulate calcification roasting process of vanadium-chromium slag[J]. Journal of Industrial and Engineering Chemistry, 2022,115:378−389. doi: 10.1016/j.jiec.2022.08.022 [17] Guo Shuanghua. Experimental study on the extraction of vanadium from ferrovanadium slag by calcification roasting and acid leaching[J]. Hydrometallurgy, 2018,37(2):111−113. (郭双华. 用钙化焙烧—酸浸法从钒铁渣中提取钒试验研究[J]. 湿法冶金, 2018,37(2):111−113.Guo Shuanghua. Experimental study on the extraction of vanadium from ferrovanadium slag by calcification roasting and acid leaching[J]. Hydrometallurgy, 2018, 37(2): 111−113. [18] Yu Tangxia, Wen Jing, Sun Hongyan, et al. Effects of phase structure of vanadium slags with different calcium content on calcified vanadium extraction[J]. Iron Steel Vanadium Titanium, 2021,42(5):18−23. (余唐霞, 温婧, 孙红艳, 等. 不同钙含量钒渣的物相结构对钙化提钒的影响[J]. 钢铁钒钛, 2021,42(5):18−23.Yu Tangxia, Wen Jing, Sun Hongyan, et al. Effects of phase structure of vanadium slags with different calcium content on calcified vanadium extraction[J]. Iron Steel Vanadium Titanium, 2021, 42(5): 18−23. [19] Wen Jing, Jiang Tao, Wang Junpeng, et al. Cleaner extraction of vanadium from vanadium-chromium slag based on MnO2 roasting and manganese recycle[J]. Joural of Cleaner Production, 2020,261(10):121205.1−121205.11. [20] Wen Jing, Jiang Tao, Wang Junpeng, et al. An efficient utilization of high chromium vanadium slag: Extraction of vanadium based on manganese carbonate roasting and detoxification processing of chromium-containing tailings[J]. Journal of Hazardous Materials, 2019,378:120733. doi: 10.1016/j.jhazmat.2019.06.010 [21] Ye Lu, Du Guangchao, Li Yuepeng, et al. Analysis of the effect of manganese on vanadium slag calcification and vanadium extraction[J]. Iron Steel Vanadium Titanium, 2022,43(6):38−44. (叶露, 杜光超, 李月鹏, 等. 锰对钒渣钙化提钒的影响分析[J]. 钢铁钒钛, 2022,43(6):38−44.Ye Lu, Du Guangchao, Li Yuepeng, et al. Analysis of the effect of manganese on vanadium slag calcification and vanadium extraction[J]. Iron Steel Vanadium Titanium, 2022, 43(6): 38−44. [22] Xiang Junyi. Optimization of vanadium extraction process by calcification of converter vanadium slag and comprehensive utilization of vanadium tailings[D]. Chongqing:Chongqing University, 2018. (向俊一. 转炉钒渣钙化提钒工艺优化及提钒尾渣综合利用基础研究[D]. 重庆:重庆大学, 2018.Xiang Junyi. Optimization of vanadium extraction process by calcification of converter vanadium slag and comprehensive utilization of vanadium tailings[D]. Chongqing: Chongqing University, 2018. -




 下载:
下载: