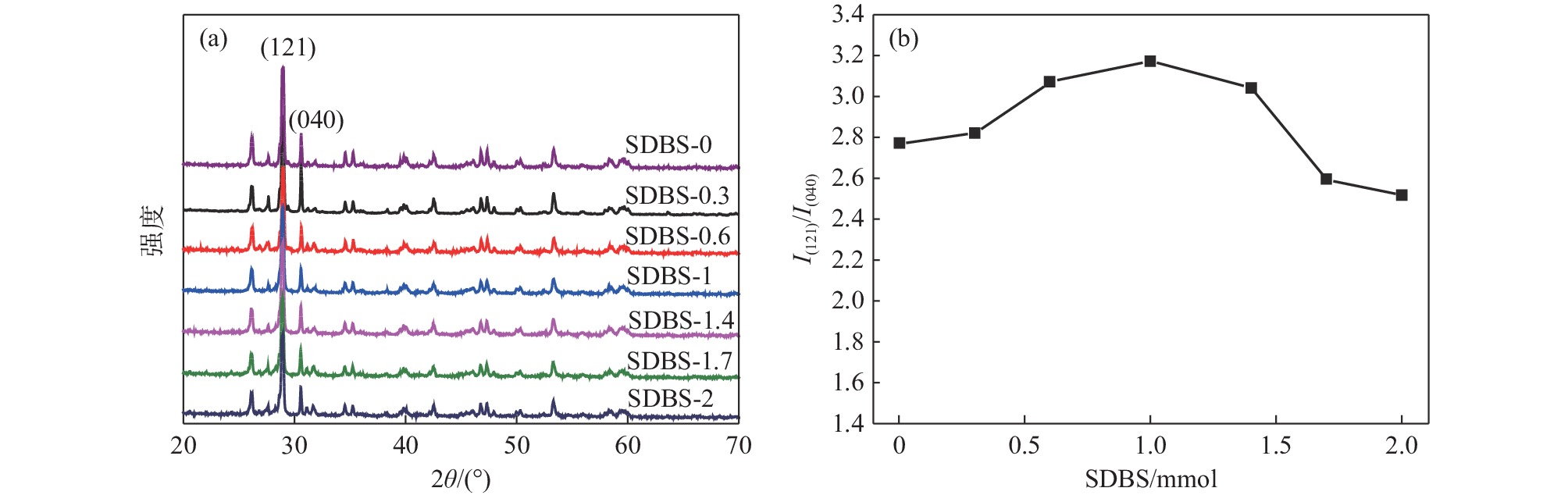
Effect of SDBS addition on properties of bismuth vanadate yellow pigment powder
-
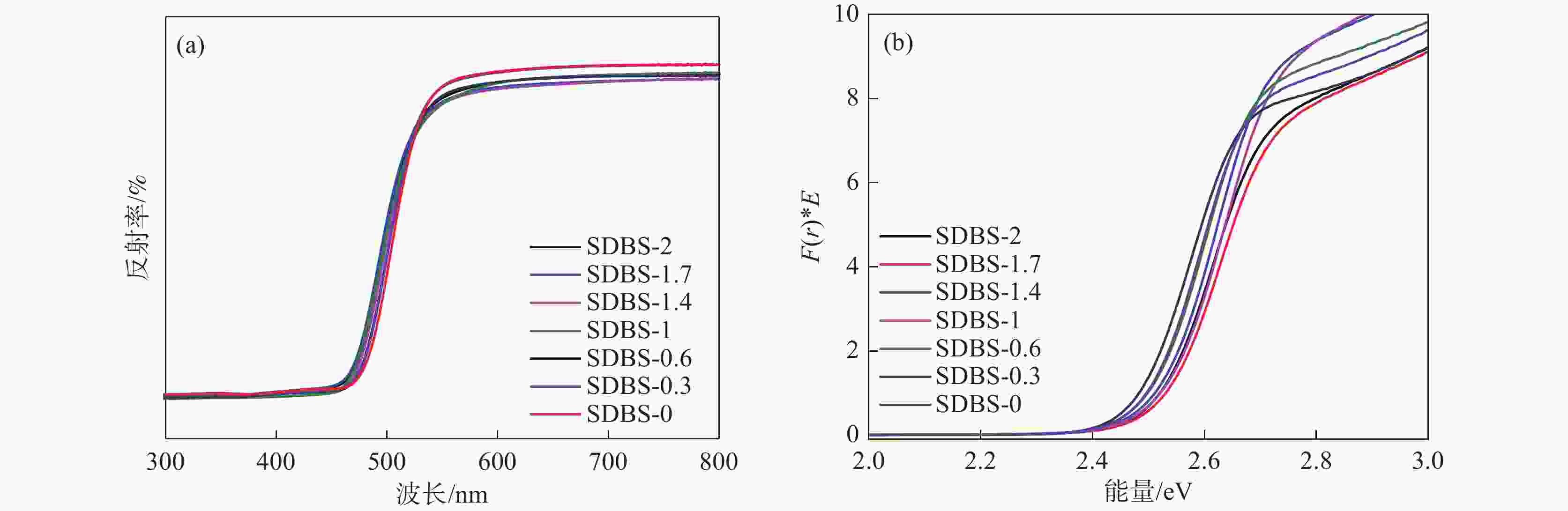
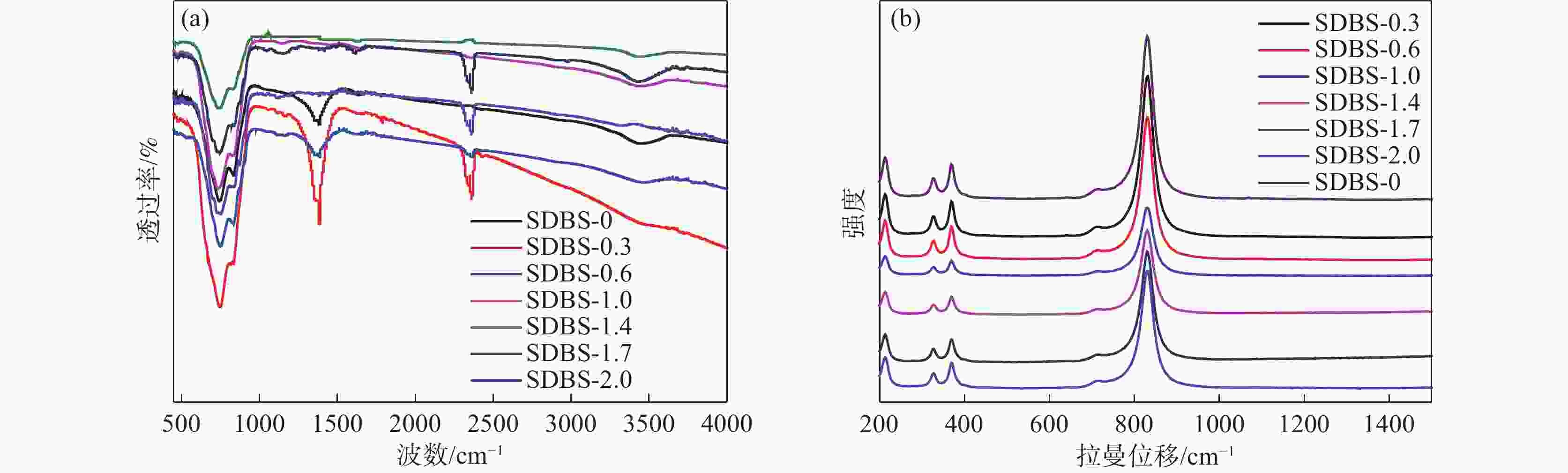
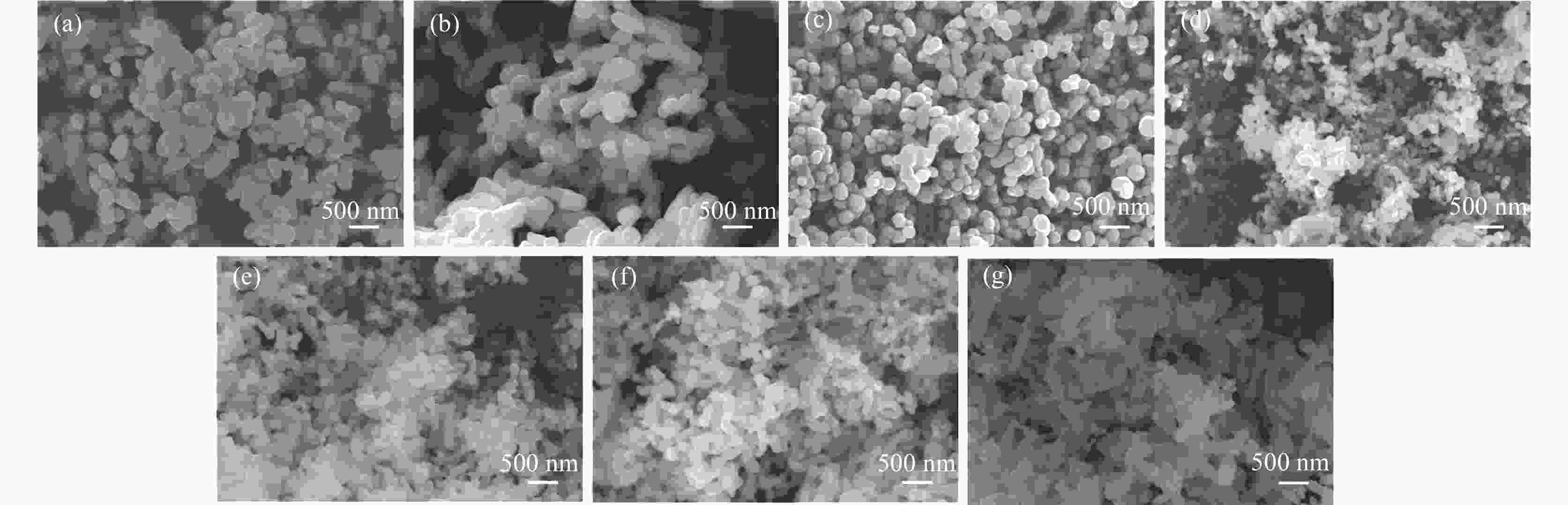
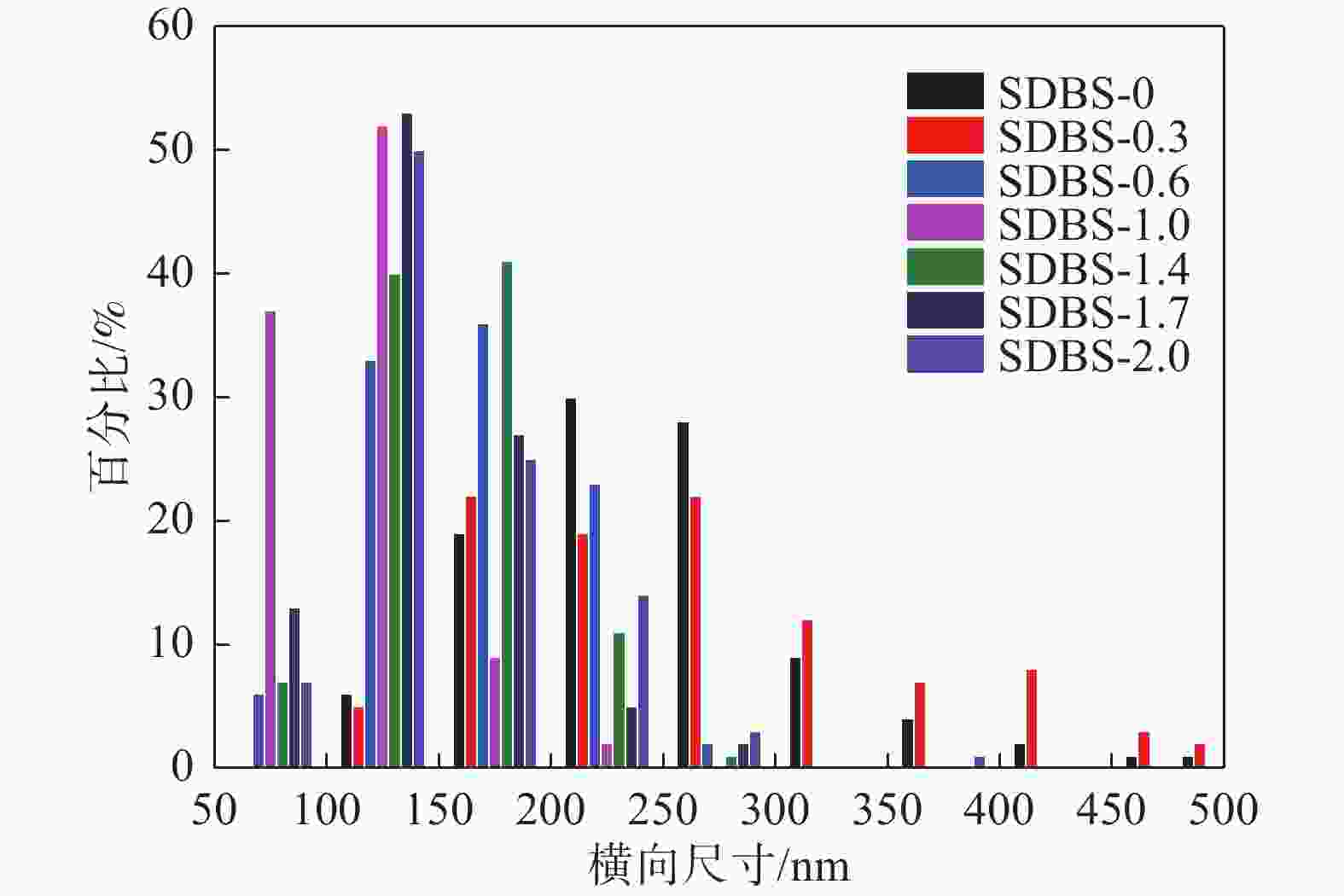
摘要: 以十二烷基苯磺酸钠(SDBS)作为结构导向剂,液相法制备钒酸铋黄色颜料纳米粉体,探究了SDBS对钒酸铋颜料形貌结构、物相以及色度值的影响。结果表明,SDBS可以在钒酸铋制备过程中促进钒酸铋(121)晶面的生长,同时,有效地降低钒酸铋颗粒尺寸,提高钒酸铋颗粒的分散稳定性,实现对钒酸铋色调的调控。SDBS添加量为1.0 mmol时,制备的钒酸铋为单斜晶系白钨矿结构,颗粒无明显团聚,平均尺寸为115.21 nm,且分布集中,89%的颗粒尺寸在50~150 nm。实现了钒酸铋由红色调黄到绿色调黄的转变,同时提高了钒酸铋的亮度L*。粉体色度参数L*=89.10,a*=−2.54,b*=71.82。Abstract: In this paper, sodium dodecylbenzene sulfonate (SDBS) was used as structure directing agent to prepare bismuth vanadate pigment nanopowder by liquid phase method.The influence of SDBS on the morphology, phase, and chromaticity value of bismuth vanadate was investigated.The results show that SDBS can promote the growth of bismuth vanadate 121 crystal plane during the preparation of bismuth vanadate. At the same time, it effectively reduces the particle size of bismuth vanadate, improves the dispersion stability of bismuth vanadate particles and achieves the regulation of the color tone of bismuth vanadate. When the amount of SDBS added is 1.0, the prepared bismuth vanadate has a monoclinic scheelite structure. The particles show no obvious agglomeration, with an average size of 115.21 nm and a concentrated distribution, with 89% of the particles ranging in the size from 50 to 150 nm. The transformation of bismuth vanadate from red to yellow to green was achieved, while the brightness L* of bismuth vanadate was improved, with a chromaticity value of L*=89.10, a*= −2.54 and b*=71.82.
-
表 1 不同SDBS添加量时制备的钒酸铋颜料的色度参数
Table 1. Colorimetric parameters of bismuth vanadate pigments prepared with different SDBS additions
SDBS添加量/mmol L* a* b* 0 87.02 1.21 72.14 0.3 88.17 −0.24 70.05 0.6 88.80 −1.12 71.22 1.0 89.10 −2.54 71.82 1.4 88.53 −2.49 71.49 1.7 88.28 −2.46 69.08 2.0 87.26 −2.34 68.29 -
[1] Zhang Heng. Preparation of bismuth yellow and its application[J]. Modern Paint and Finishing, 2019,22(4):27−30. (张亨. 铋黄的制备和应用[J]. 现代涂料与涂装, 2019,22(4):27−30.Zhang Heng. Preparation of bismuth yellow and its application[J]. Modern Paint and Finishing, 2019, 22(4): 27−30. [2] Slobodan D Dolic, Dragana J Jovanovic, Krisjanis Smits, et al. A comparative study of photocatalytically active nanocrystalline tetragonal zyrcon-type and monoclinic scheelite-type bismuth vanadate[J]. Ceramics International, 2018,44:17953−17961. doi: 10.1016/j.ceramint.2018.06.272 [3] Yiseul Park, Kenneth J McDonald, Kyoung-Shin Choi. Progress in bismuth vanadate photoanodes for use in solar water oxidation[J]. Chemical Society Reviews, 2013,42(6):2321−2337. doi: 10.1039/C2CS35260E [4] Elisabeth G van der Linden, Luiz Fernando B Malta, Marta Eloisa Medeiros. Evaluation of synthetic routes to pigmentary grade bismuth vanadate[J]. Dyes & Pigments, 2011,90(1):36−40. [5] Li Jinqi, Guo Zhi, Liu Yanxiu. Effect of grinding time on the photocatalytic performance of BiVO4 prepared by solid phase method[J]. Speciality Petrochemicals, 2022, 39(3): 26−29. (李金琦, 郭智, 柳艳修. 研磨时间对固相法制备钒酸铋光催化性能的影响[J]. 精细石油化工,2022, 39(3): 26−29.Li Jinqi, Guo Zhi, Liu Yanxiu. Effect of grinding time on the photocatalytic performance of BiVO4 prepared by solid phase method[J]. Speciality Petrochemicals, 2022, 39(3): 26−29. [6] Du Guangchao, Yin Danfeng, Sun Zhaohui, et al. Preparation of bismuth vanadate pigment by solid-state calcination[J]. Iron Steel Vanadium Titanium, 2016,37(5):35−42. (杜光超, 尹丹凤, 孙朝晖, 等. 固相煅烧法制备钒酸铋颜料工艺研究[J]. 钢铁钒钛, 2016,37(5):35−42.Du Guangchao, Yin Danfeng, Sun Zhaohui, et al. Preparation of bismuth vanadate pigment by solid-state calcination[J]. Iron Steel Vanadium Titanium, 2016, 37(5): 35−42. [7] Du Guangchao, Sun Zhaohui, Xian Yong, et al. Kinetics of BiVO4 prepared by solid reaction between Bi(NO3)3·5H2O and and NH4VO3 under calcination[J]. Iron Steel Vanadium Titanium, 2015,36(5):34−39. (杜光超, 孙朝晖, 鲜勇, 等. 固相煅烧法制备钒酸铋的动力学研究[J]. 钢铁钒钛, 2015,36(5):34−39.Du Guangchao, Sun Zhaohui, Xian Yong, et al. Kinetics of BiVO4 prepared by solid reaction between Bi(NO3)3·5H2O and and NH4VO3 under calcination[J]. Iron Steel Vanadium Titanium, 2015, 36(5): 34−39. [8] Andreas Tucks, Horst P Beck. The photochromic effect of bismuth vanadate pigments: Investigations on the photochromic mechanism[J]. Dyes & Pigments, 2007,72(2):163−177. [9] Tao Rui, Zhang Xiaozhen, Liu Huafeng, et al. Effect of Zn doping on the properties of nano BiVO4 yellow pigment[J]. Journal of Synthetic Crystals, 2019,48(6):1144−1149. (陶锐, 张小珍, 刘华锋, 等. Zn掺杂量对纳米BiVO4黄色颜料性能的影响[J]. 人工晶体学报, 2019,48(6):1144−1149.Tao Rui, Zhang Xiaozhen, Liu Huafeng, et al. Effect of Zn doping on the properties of nano BiVO4 yellow pigment[J]. Journal of Synthetic Crystals, 2019, 48(6): 1144−1149. [10] Tao Rui, Jiang Yuhua, Liu Huafeng, et al. Effctes of metal ions doping on the chromatic performance of BiVO4 yellow pigments[J]. Journal of Ceramics, 2019,40(3):372−376. (陶锐, 江瑜华, 刘华锋, 等. 金属离子掺杂对BiVO4黄色颜料性能的影响[J]. 陶瓷学报, 2019,40(3):372−376.Tao Rui, Jiang Yuhua, Liu Huafeng, et al. Effctes of metal ions doping on the chromatic performance of BiVO4 yellow pigments[J]. Journal of Ceramics, 2019, 40(3): 372−376. [11] Zhao Guosheng, Liu Wei, Hao Yan, et al. Nanostructured shuriken-like BiVO4 with preferentially exposed {010} facets: preparation, formation mechanism, and enhanced photocatalytic performance[J]. Dalton Transactions An International Journal of Inorganic Chemistry, 2018,47(4):1325−1336. [12] Yu Xinxin, Zhang Xiufang, Li Zhipeng. Preparation of BiVO4 photocatalyst with different morphologies and its photocatalytic performance[J]. Journal of Dalian Polytechnic University, 2020,39(3):188−192. (于欣鑫, 张秀芳, 李志鹏. 不同形貌BiVO4光催化剂的制备及其光催化性能[J]. 大连工业大学学报, 2020,39(3):188−192.Yu Xinxin, Zhang Xiufang, Li Zhipeng. Preparation of BiVO4 photocatalyst with different morphologies and its photocatalytic performance[J]. Journal of Dalian Polytechnic University, 2020, 39(3): 188−192. [13] Ahmed Helal, Said M El-Sheikh, Jianqiang Yu, et al. Novel synthesis of BiVO4 using homogeneous precipitation and its enhanced photocatalytic activity[J]. Journal of Nanoparticle Research, 2020,22(132):1−11. [14] Shan Lianwei, Lu Changhui, Dong Limin, et al. Efficient facet regulation of BiVO4 and its photocatalytic motivation[J]. Journal of Alloys and Compounds, 2019,804:385−391. doi: 10.1016/j.jallcom.2019.07.051 [15] Li Zhaosheng, Luo Wenjun, Zhang Minglong, et al. Photoelectrochemical cells for solar hydrogen production: current state of promising photoelectrodes, methods to improve their properties, and outlook[J]. Energy & Environmental Science, 2013,6(2):347−370. [16] Nagabhushana G P, Tavakoli A H, Navrotsky A. Energetics of bismuth vanadate[J]. Journal of Solid State Chemistry, 2015,225(2):187−192. [17] Lin Xue, Li Hongji, Yu Lili, et al. Efficient removal rhodamine B over hydrothermally synthesized fishbone like BiVO4[J]. Materials Research Bulletin, 2013,48(10):4424−4429. doi: 10.1016/j.materresbull.2013.06.075 [18] Wang Xiaowen, Mu Bin, Hui Aiping, et al. Low-cost bismuth yellow hybrid pigments derived from attapulgite[J]. Dyes and Pigments, 2018,149:521−530. doi: 10.1016/j.dyepig.2017.10.041 [19] L Sandhya Kumari, P Prabhakar Rao, A Narayana Pillai Radhakrishnan, et al. Brilliant yellow color and enhanced NIR reflectance of monoclinic BiVO4 through distortion in VO4 tetrahedra[J]. Solar Energy Materials & Solar Cells, 2013,112:134−143. [20] Mohamad Fakhrul, Ridhwan Samsudin, Suriati Sufian, et al. Synergistic effects of pH and calcination temperature on enhancing photodegradation performance of m-BiVO4[J]. Journal of the Taiwan Institute of Chemical Engineers, 2017,81:305−315. doi: 10.1016/j.jtice.2017.09.045 -




 下载:
下载: