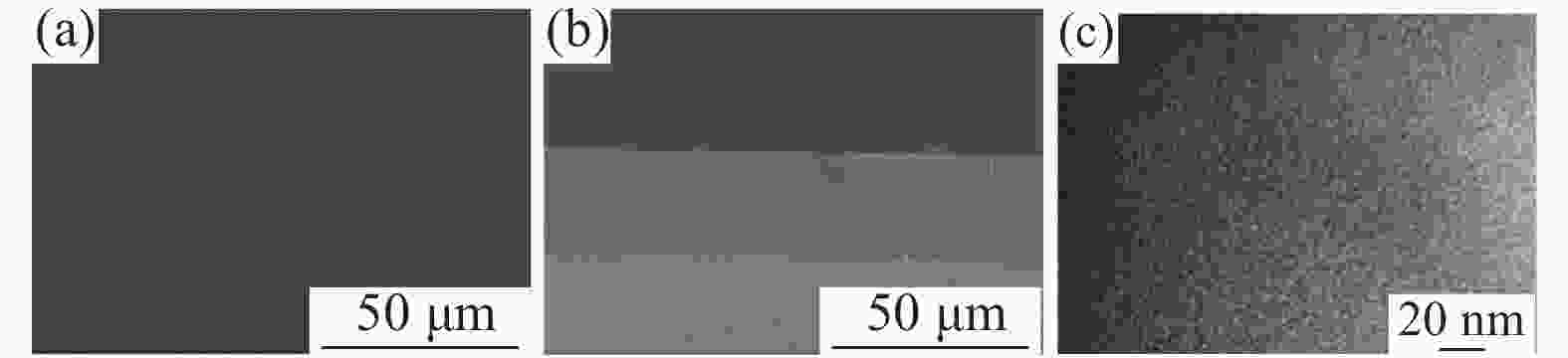
Preparation and properties of polyvinylpyrrolidone & polyvinyl chloride composite proton exchange membrane for vanadium redox flow batteries
-
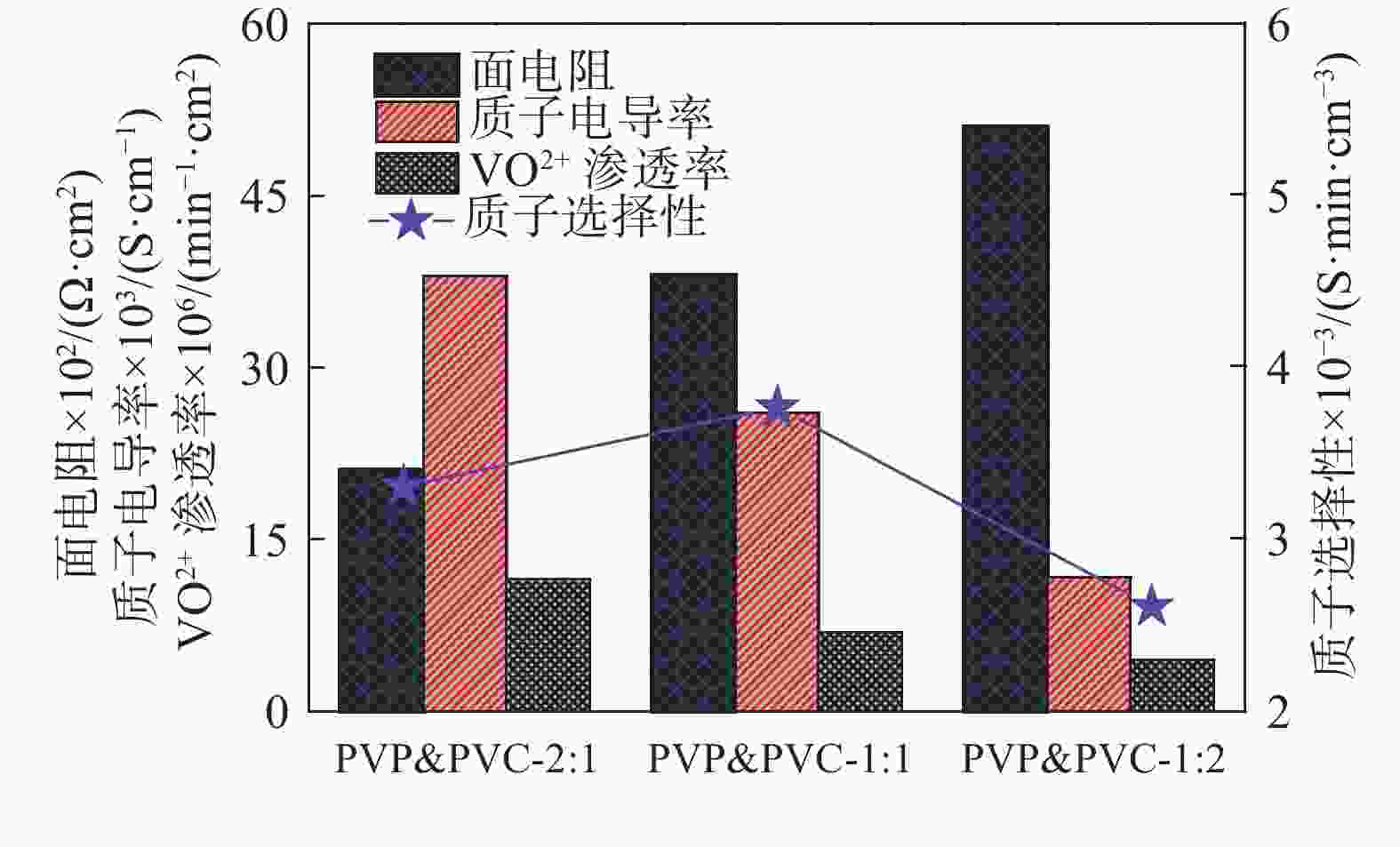
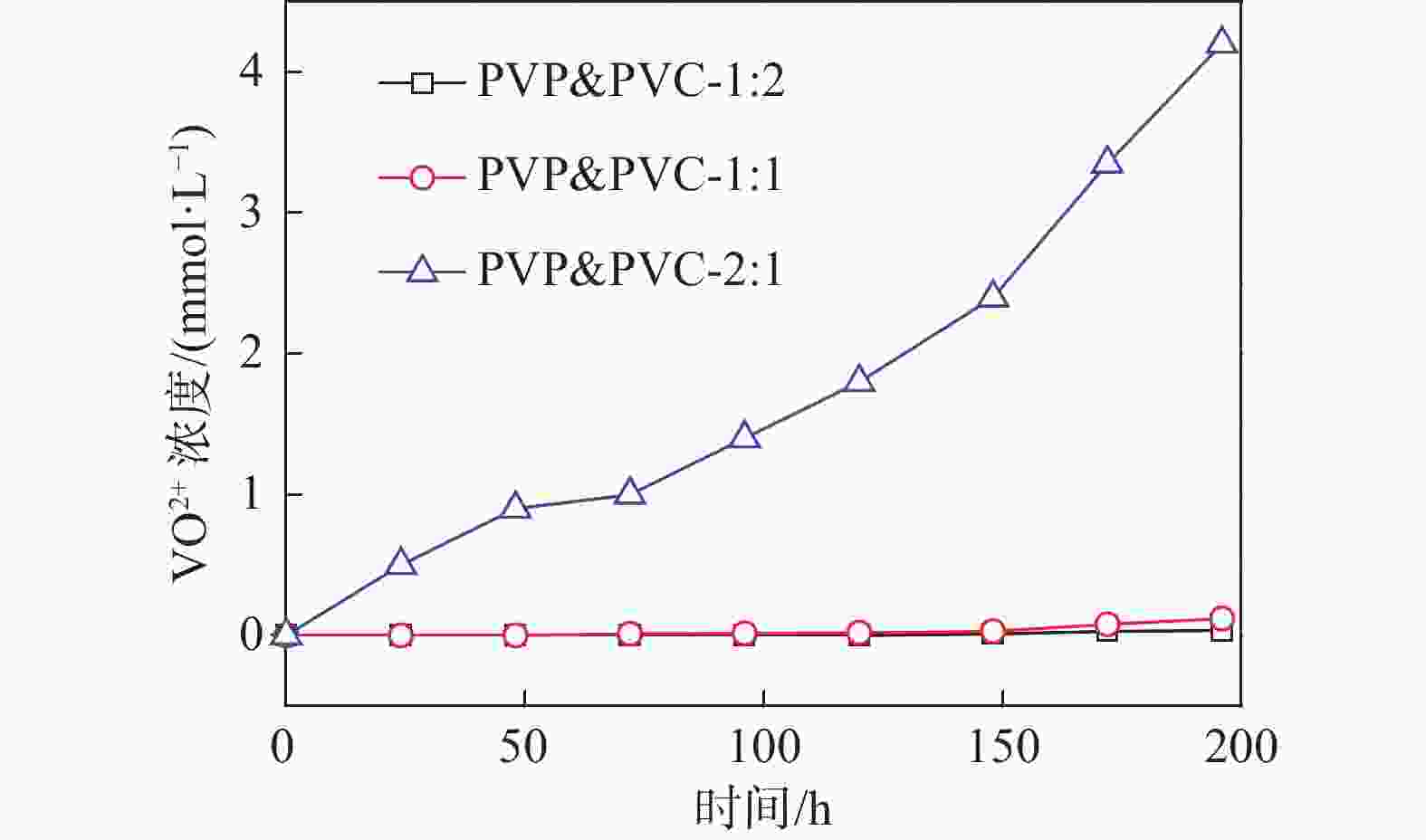
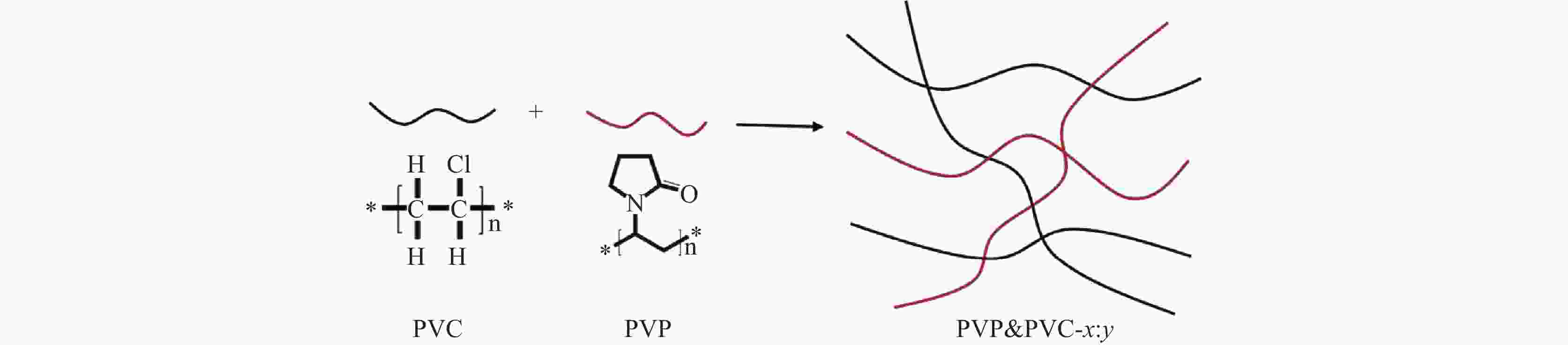
摘要: 质子交换膜(PEM)作为全钒氧化还原液流电池(VRFB)的关键组成部分,在控制VRFB的性能以及成本方面起着重要的作用。制备了一系列不同比例的聚乙烯吡咯烷酮(PVP)&聚氯乙烯(PVC)复合质子交换膜,其中PVP由于胺基的质子化作用,在膜中进行离子传导,而PVC作为骨架起到支撑作用。通过调整复合膜中PVP和PVC的比例,得到性能适用于全钒液流电池的质子交换膜。研究发现,PVP&PVC复合膜中PVC含量增加,膜的机械性能增强,钒离子透过率降低;而膜中PVP含量增加,膜的电导率和溶胀随之变大。测试结果表明,当复合膜中PVP和PVC质量比为1:1时,混合膜的质子选择性最高为3.8×103 S·min·cm−3,复合膜综合性能最好,并且在电流密度为50 mA·cm−2时,该单电池具有较高的充放电容量。
-
关键词:
- 全钒液流电池 /
- 质子交换膜 /
- 聚乙烯吡咯烷酮 /
- 聚氯乙烯;质子选择性
Abstract: Proton exchange membrane (PEM), as a key component of vanadium redox flow battery (VRFB), plays an important role in controlling the performance and cost of VRFB. A series of PVP&PVC composite PEMs were prepared, wherein PVP carried out ion conduction in the membrane due to the protonation of amine groups, and PVC acted as skeleton to support the membrane. By adjusting the ratio of PVP and PVC in the composite membrane, the PEM which is suitable for all-vanadium flow battery was obtained. It is found out that with the increase of PVC content in the composite PEM, the mechanical property of the membrane is enhanced, and the transmittance of vanadium ion is decreased. The conductivity and swelling of the membrane increased with the increase of PVP content. The results show that when the mass ratio of PVP and PVC in the composite membrane is 1:1, the proton selectivity is 3.8×103 S·min·cm–3 which is the highest among the investigated composite membranes, so the comprehensive performance of PVP&PVC-1:1 composite membrane is the best. When the current density is 50 mA·cm–2, the single battery assembled with PVP&PVC-1:1 membrane is possessed a good charge and discharge capacity. -
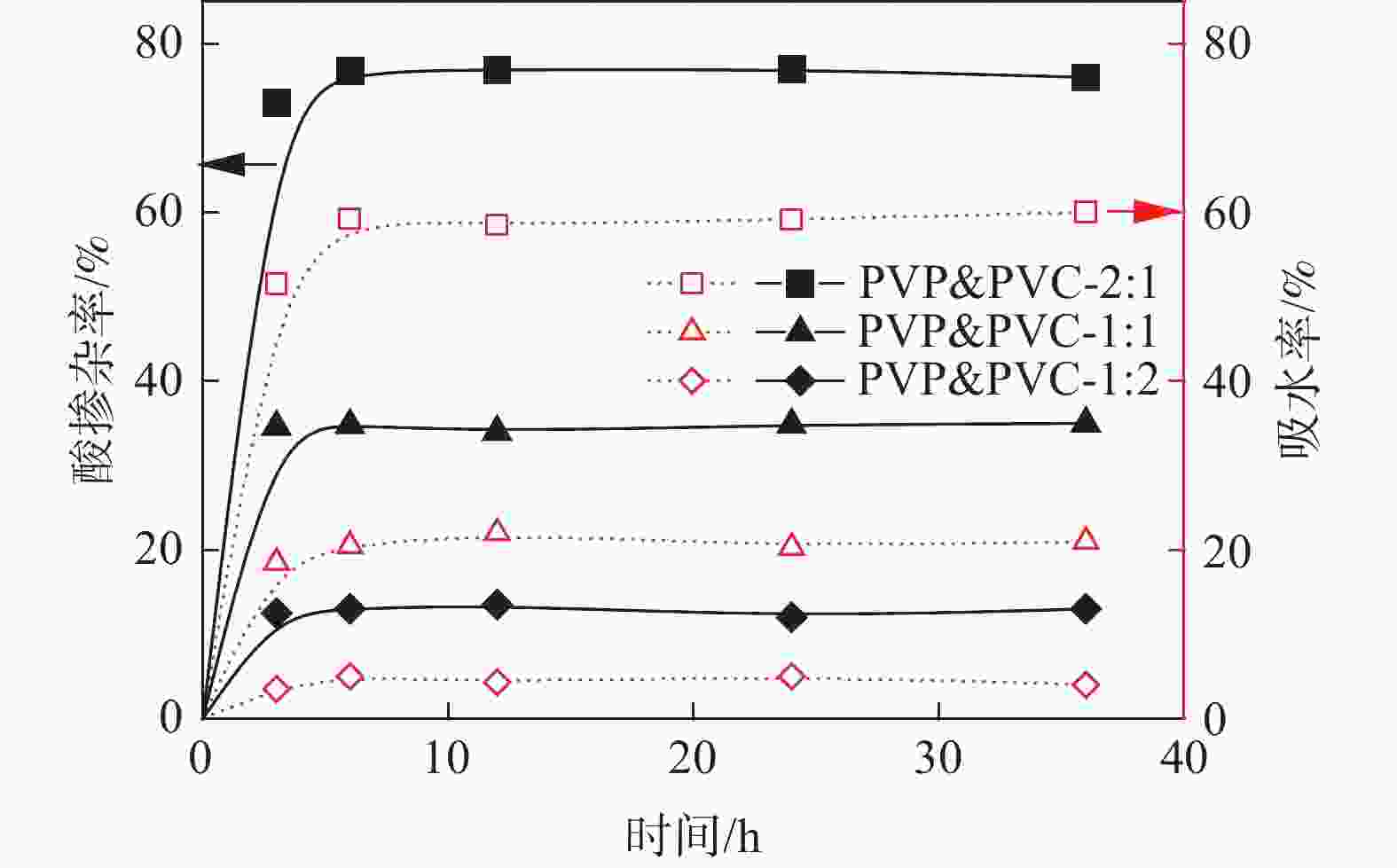
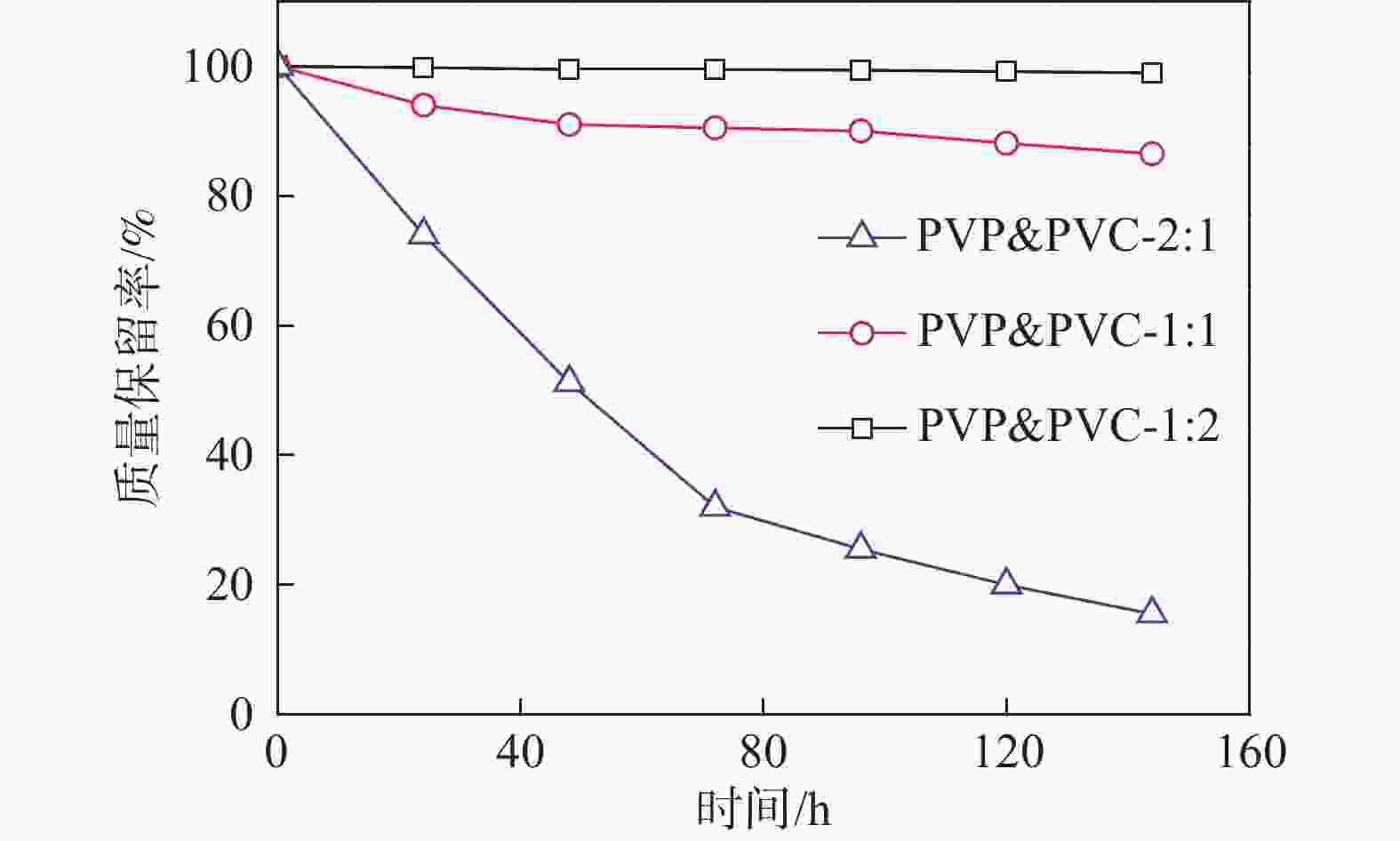
表 1 PVP&PVC-x:y膜的吸水率、酸掺杂率和溶胀
Table 1. The Water uptake, acid doping and swelling of membranes
% 膜样品 浸入水中 浸入酸中 吸水率 面积溶胀 体积溶胀 酸掺杂率 面积溶胀 体积溶胀 PVP&PVC-2:1 59.5 42.3 63.2 76.9 53.1 83.0 PVP&PVC-1:1 19.3 12.2 26 34.8 35.2 73.0 PVP&PVC-1:2 5.2 2.9 5.2 13.0 14.2 25.4 表 2 酸掺杂的PVP&PVC-x:y膜的机械性能
Table 2. Mechanical properties of the PVP&PVC-x:y membranes doped in 3 mol/L SA solution
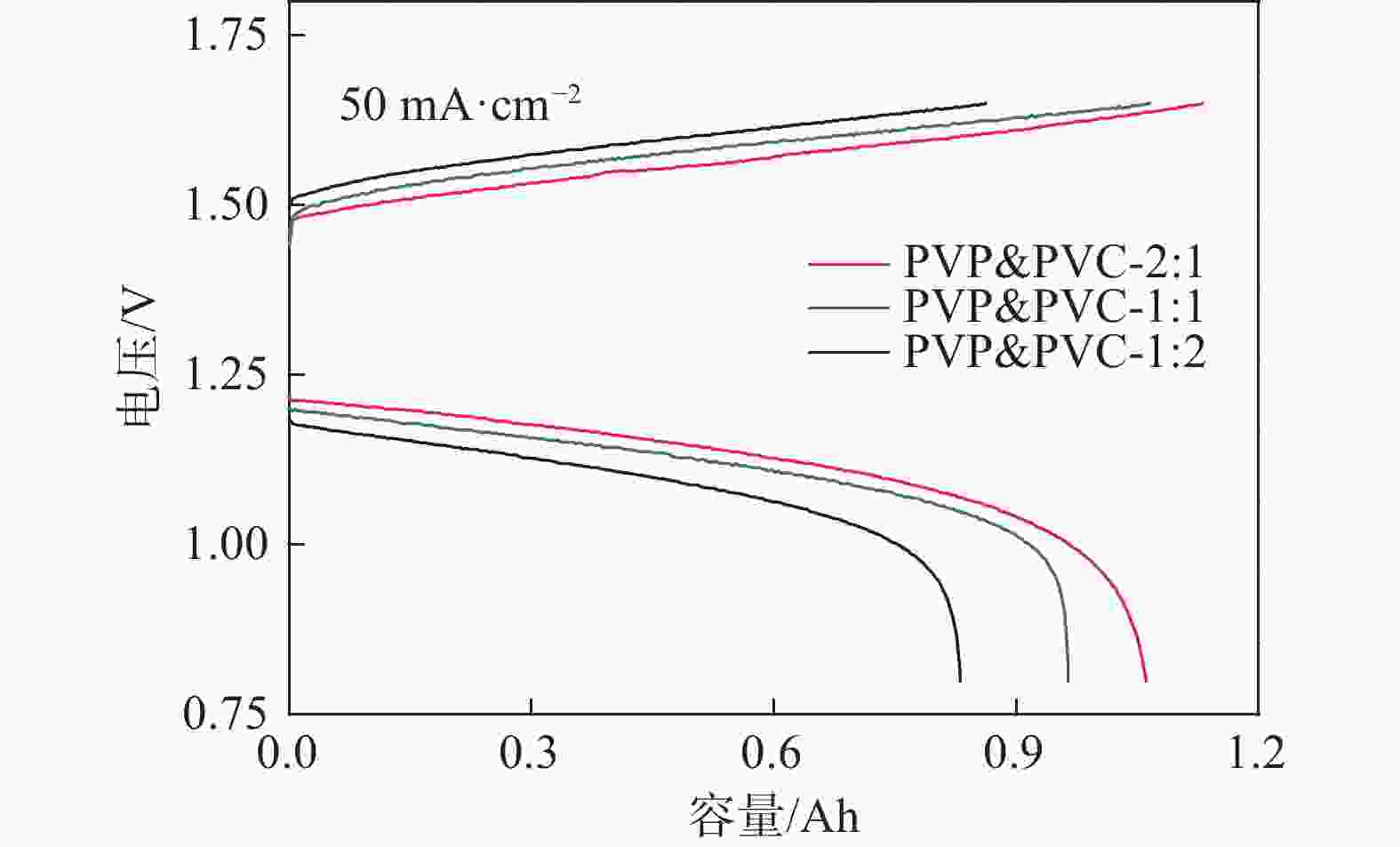
膜样品 拉伸强度/MPa 断裂伸长率/% SA/PVP&PVC-2:1 13.2±1.5 120.0±2.9 SA/PVP&PVC-1:1 30.1±2.5 105.6±3.8 SA/PVP&PVC-1:2 36.5±1.9 72.2±3.6 表 3 PVP&PVC-1:1膜在不同电流密度下的电池性能
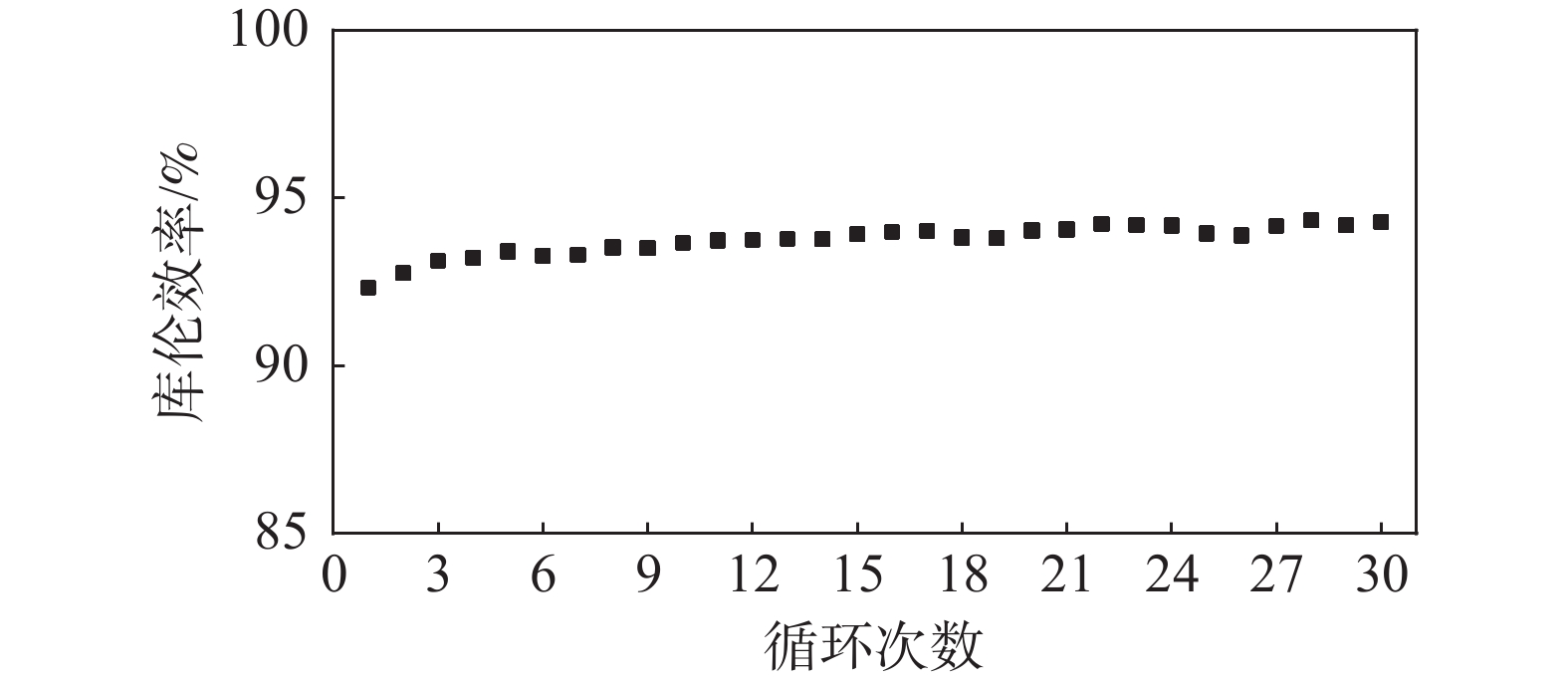
Table 3. Cell performance assembled with PVP&PVC-1:1 membrane at various current densities
电流密度/(mA·cm−2) CE/% VE/% EE/% 20 83.0 89.9 74.6 50 92.7 86.5 80.2 100 94.9 83.2 79.0 -
[1] Gao J, Chen H, Li Y, et al. Fuel consumption and exhaust emissions of diesel vehicles in worldwide harmonized light vehicles test cycles and their sensitivities to eco-driving factors[J]. Energy Conversion and Management, 2019,196:605-613. doi: 10.1016/j.enconman.2019.06.038 [2] Pursiheimo E, Holttinen H, Koljonen T. Inter-sectoral effects of high renewable energy share in global energy system[J]. Renewable Energy, 2019,136:1119-1129. [3] Zhang Chao, Wei Yili, Cao Pengfei, et al. Energy storage system: Current studies on batteries and power condition system [J]. Renewable & Sustainable Energy Reviews, 2018, 82(3): 3091-3106. [4] Thaller L H. Electrically rechargeable Redox flow cell[C]//9th Intersociety Energy Conversion Engineering Conference, 1976. doi: US3996064 A. [5] Yon Ruiting, Wang Qing. Redox-targeting-based flow batteries for large-scale energy storage[J]. Advanced Materials, 2018, 30(47):1802406.1-13. [6] Shi Yu, Eze Chika, Xiong Binyu, et al. Recent development of membrane for vanadium redox flow battery applications: A review[J]. Applied Energy, 2019,238:202-224. doi: 10.1016/j.apenergy.2018.12.087 [7] Lee M S, Kang H G, Jeon J D, et al. A novel amphoteric ion-exchange membrane prepared by the pore-filling technique for vanadium redox flow batteries[J]. RSC Adv, 2016,6(67):63023-63029. doi: 10.1039/C6RA07790K [8] Mohammadi T, Skyllaskazacos M. Preparation of sulfonated composite membrane for vanadium redox flow battery applications[J]. J Membr Sci, 1995,107(1-2):35-45. doi: 10.1016/0376-7388(95)00096-U [9] Schwenzer B, Zhang J, Kim S, et al. Membrane development for vanadium redox flow batteries[J]. Chem Sus Chem, 2021,4(10):1388-1406. [10] Wu Chunxiao, Lu Shanfu, Wang Haining, et al. A novel polysulfone-polyvinylpyrrolidone membrane with superior proton-to-vanadium ion selectivity for vanadium redox flow batteries[J]. Journal of Materials Chemistry A, 2016,4(4):1174-1179. doi: 10.1039/C5TA08593D [11] Li Anfeng, Wang Gang, Wei Xiaoyan, et al. Highly selective sulfonated poly(ether ether ketone)/polyvinylpyrrolidone hybrid membranes for vanadium redox flow batteries[J]. Journal of Materials Science, 2020,55(35):1-14. [12] Zhang Qi, Dong Quanfeng, Zheng Mingsen, et al. The preparation of a novel anion-exchange membrane and its application in all-vanadium redox batteries[J]. Journal of Membrane Science, 2012,421-422:232-237. [13] Wang Shengyao, Fang Lifeng, Cheng Liang, et al. Novel ultrafiltration membranes with excellent antifouling properties and chlorine resistance using a poly(vinyl chloride)-based copolymer[J]. Journal of Membrane Science, 2018,549: 101-110. [14] Yong Ming, Zhang Yuqing, Sun Shuai, et al. Properties of polyvinyl chloride (PVC) ultrafiltration membrane improved by lignin: Hydrophilicity and antifouling[J]. Journal of Membrane Science, 2019,575:50-59. doi: 10.1016/j.memsci.2019.01.005 [15] Li Mei, Han Li. General chemistry[M]. Shanghai: Shanghai Jiao Tong University Press, 2015. (李梅, 韩莉. 普通化学[M]. 上海: 上海交通大学出版社, 2015.Li Mei, Han Li. General chemistry[M]. Shanghai: Shanghai Jiao Tong University Press, 2015. [16] Dai Yu, Wang Jin, Tao Peipei, et al. Various hydrophilic carbon dots doped high temperature proton exchange composite membranes based on polyvinylpyrrolidone and polyethersulfone[J]. Journal of Colloid and Interface Science, 2019,553:503-511. doi: 10.1016/j.jcis.2019.06.020 -




 下载:
下载: