Study on the influences of melt components on the physical properties of molten salt chlorination system
-
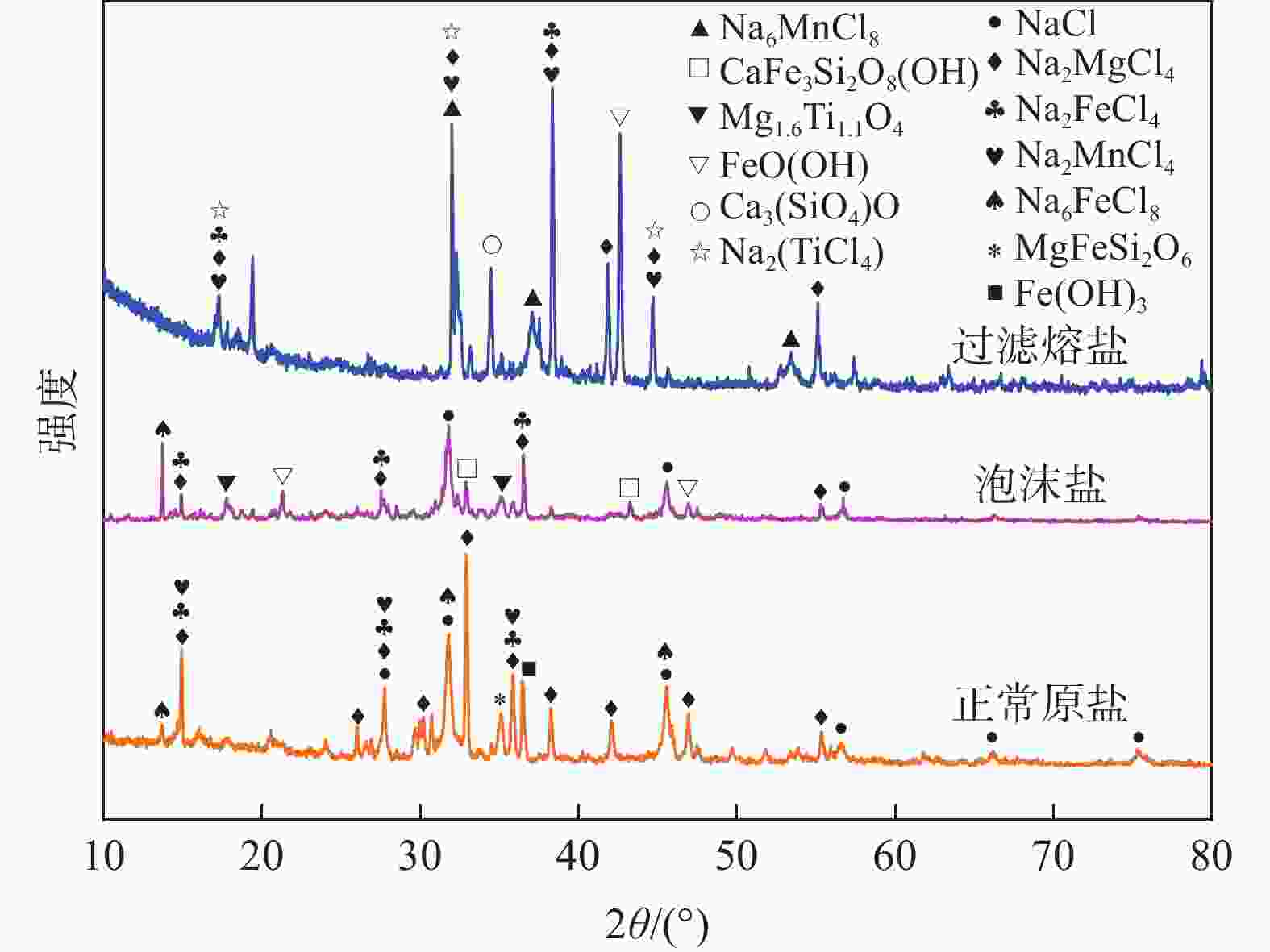
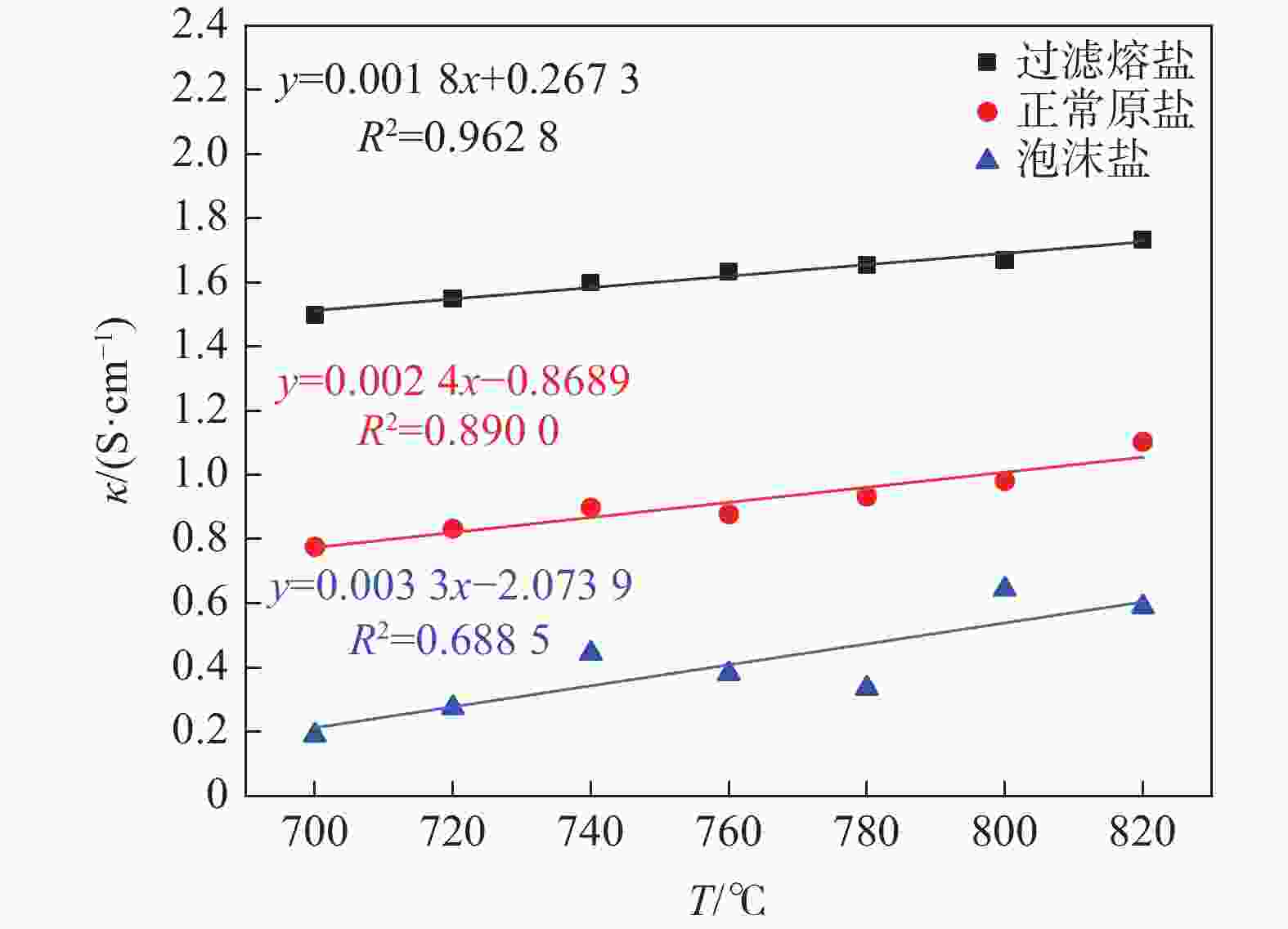
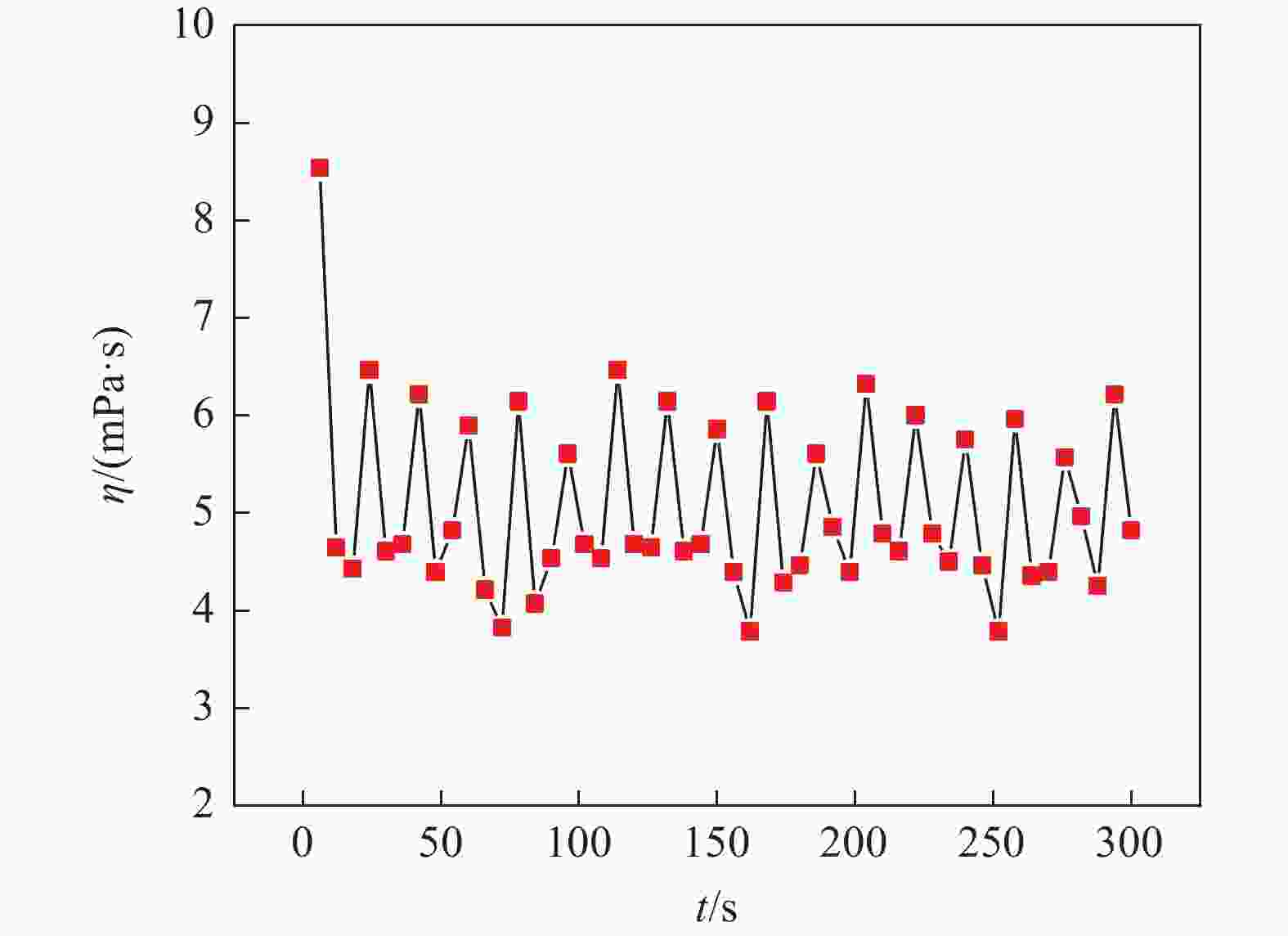
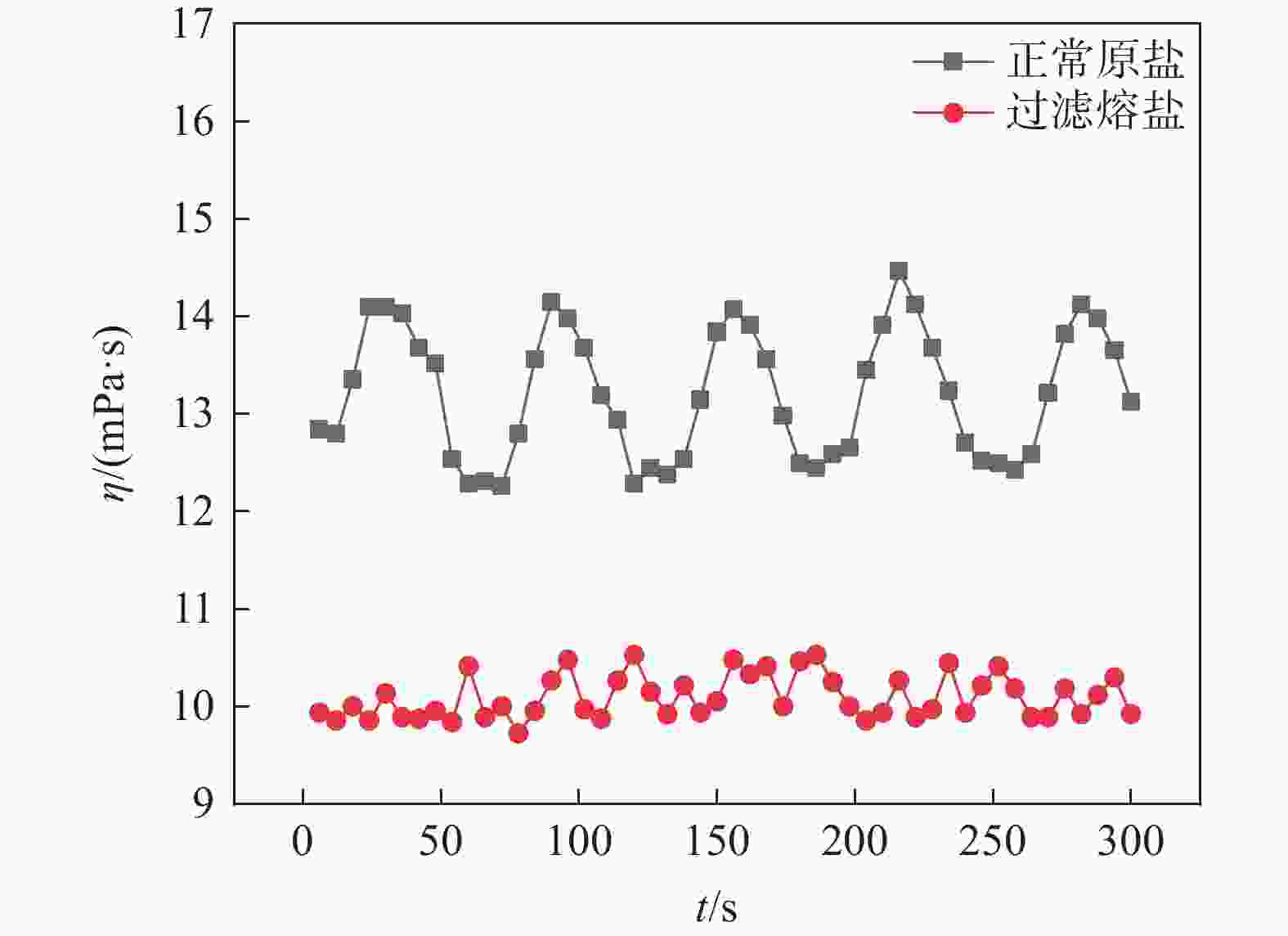
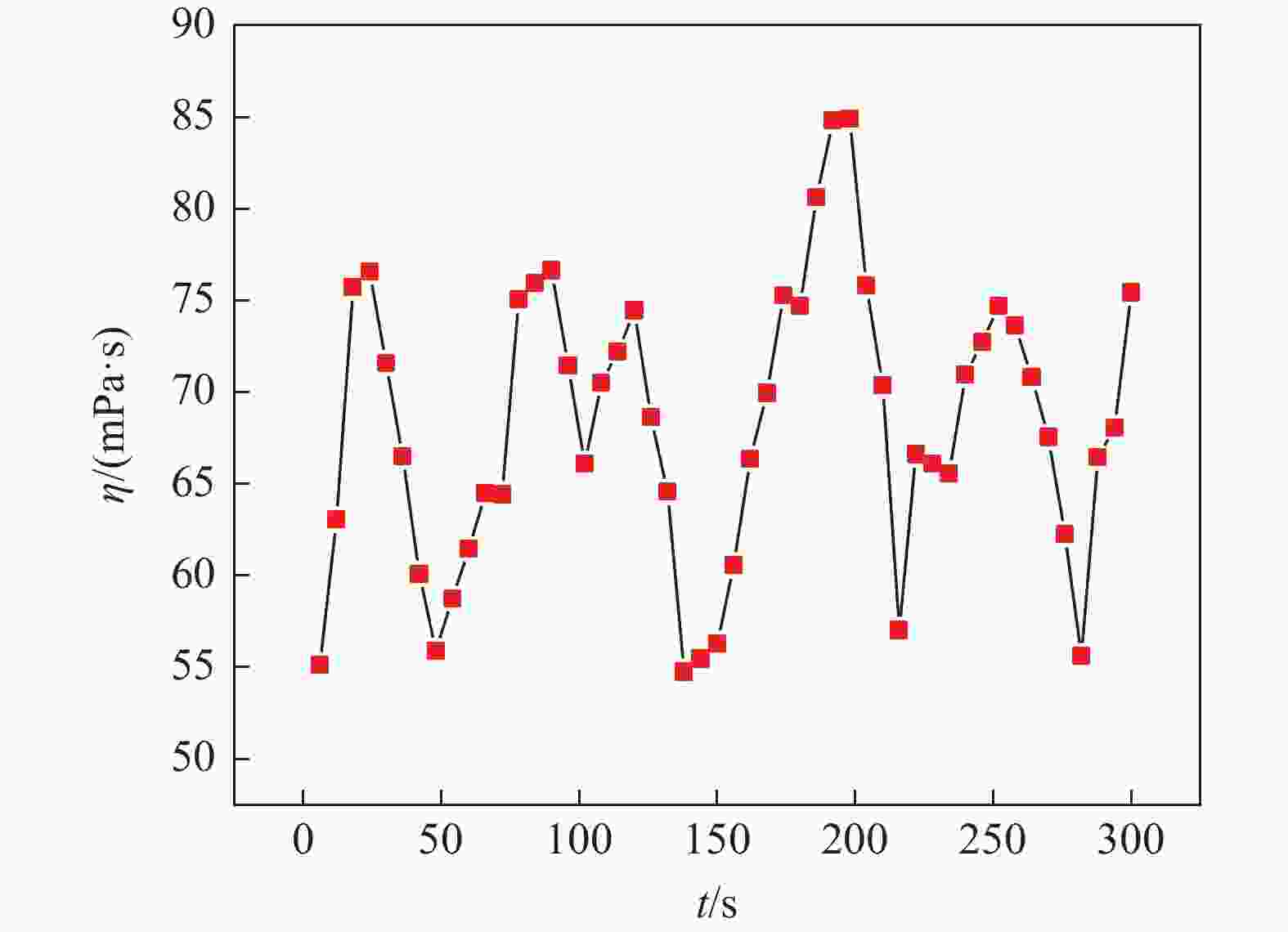
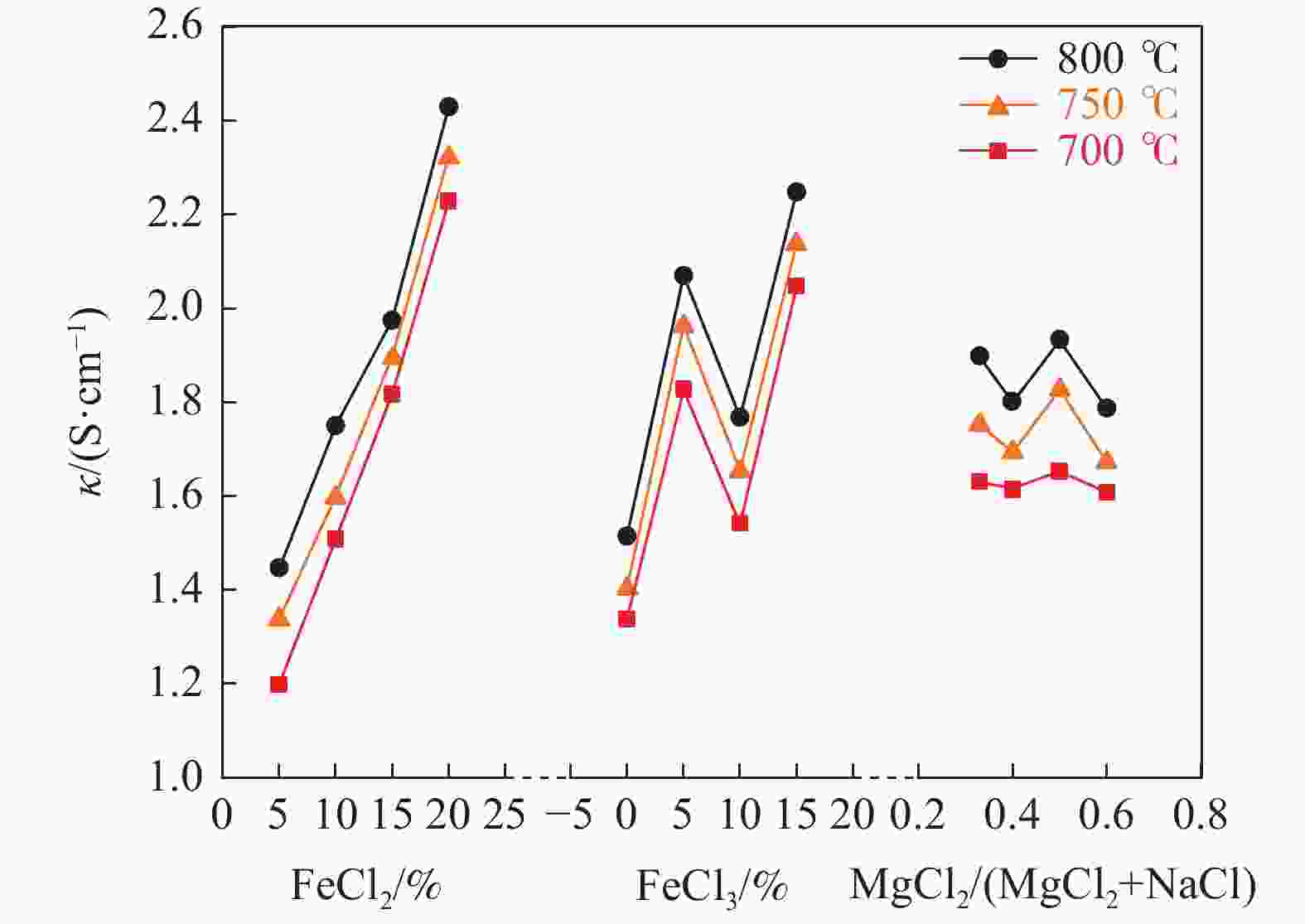
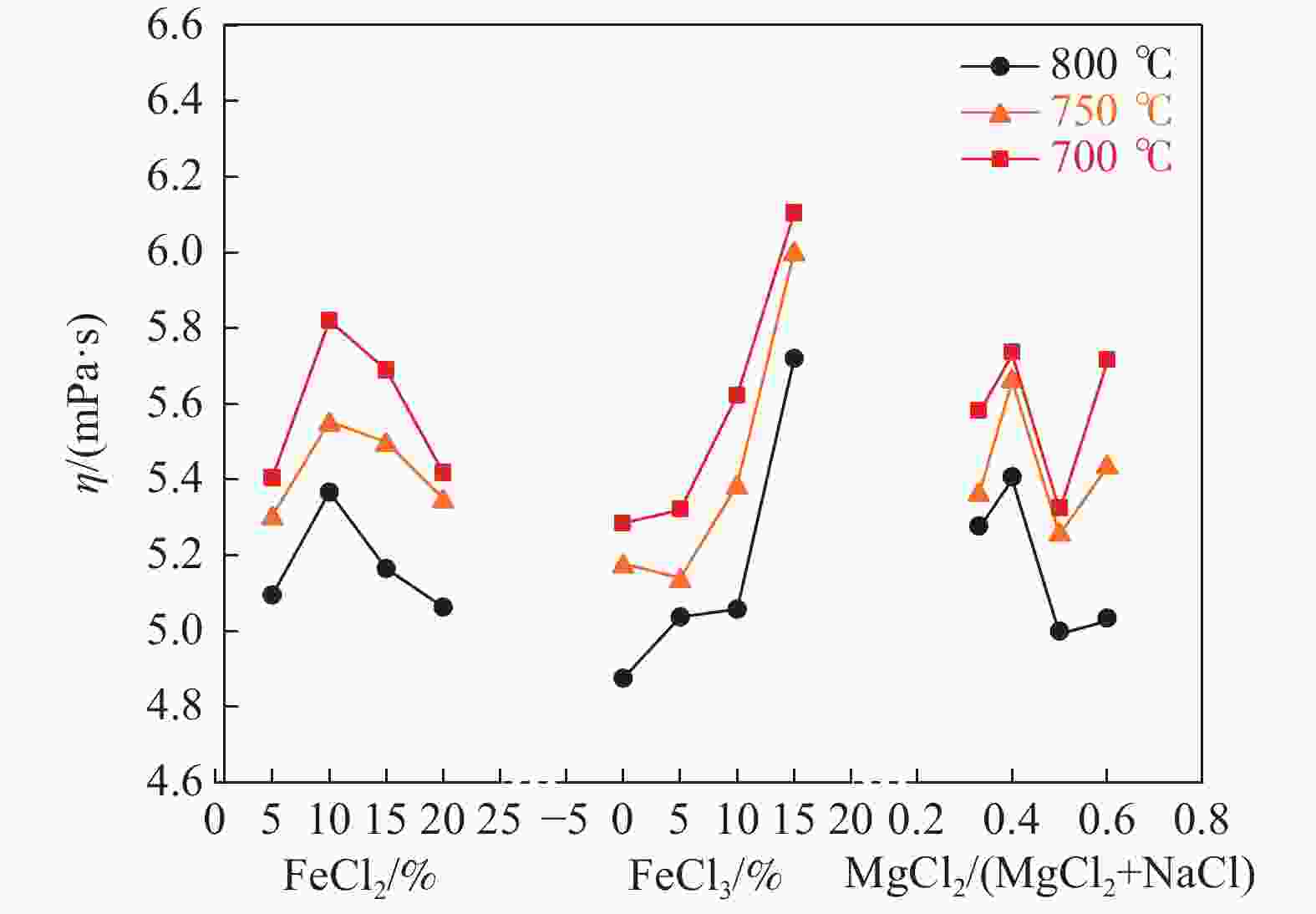
摘要: 氯化物熔盐物性对于熔盐氯化法制备四氯化钛效率至关重要。为了考察氯化用熔盐组分含量变化对熔盐体系物性的影响规律,以工业生产中的熔盐为对象,对正常原盐、泡沫盐和过滤熔盐的电导率和粘度物性进行了测试以及XRD物相分析、化学成分分析。根据实际生产中的组分配比以及化学分析结果,设置了三因素为FeCl2、FeCl3以及MgCl2 : NaCl的正交试验,研究不同组分配比下熔盐体系的物性变化。结果表明:FeCl2含量对熔盐体系电导率影响最为显著,每5%的FeCl2增加量会使熔盐整体电导率提高0.33 S/cm;FeCl3含量对熔盐体系粘度影响最大,其组分占比在10 %~ 15%区间内的影响最为明显。最后,利用综合评分法评估出最佳的熔盐氯化组分方案为:CaCl2 4%、FeCl2 20%、FeCl3 5%、MgCl2 : NaCl = 1 : 1。Abstract: The physical properties of chloride molten salt are very important for the preparation efficiency of titanium tetrachloride produced by molten salt chlorination. To investigate the influence of changes in chloride-containing molten salt composition on the physical properties of molten salt systems, this study conducted the physical property tests including conductivity and viscosity on normal raw salt, foam salt and filtered molten salt. Furthermore, XRD analysis and chemical composition analysis were also performed. Based on the component ratios in actual production and chemical analysis results, an orthogonal experiment with three factors including FeCl2, FeCl3, and MgCl2 : NaCl was designed to study the changes of physical properties of molten salt systems under different component ratios. The results indicate that the FeCl2 content has the most significant effect on the conductivity of the molten salt system, with an increase of 5% FeCl2 resulting in a 0.33 S/cm increase in overall molten salt conductivity. The FeCl3 content has the greatest impact on the viscosity of the molten salt system, with the effect being most pronounced within the 10% to 15% range of its composition. Finally, utilizing a comprehensive scoring method, the optimal chlorinated component scheme for molten salt was determined to be CaCl2 4%, FeCl2 20%, FeCl3 5% and MgCl2 : NaCl = 1 : 1.
-
Key words:
- TiCl4 /
- molten salt chlorination /
- melt components /
- normal raw salt /
- foam salt /
- filtered molten salt /
- electrical conductivity /
- viscosity
-
表 1 试剂样品信息
Table 1. Reagent sample information
试剂名称 纯度(AR)/% 品牌 氯化钠 ≥99.5 Greagent 无水氯化镁 99.5 益之辰 氯化钙 99.0 阿拉丁 无水氯化亚铁 99.5 麦克林 无水氯化铁 99.5 麦克林 表 2 正交试验因素及水平
Table 2. Orthogonal experimental factors and horizontal parameters
水平 因素 A
FeCl2含量/%B
FeCl3含量/%C
L = MgCl2 : NaCl1 5 0 3 : 2 2 10 5 1 : 1 3 15 10 2 : 3 4 20 15 1 : 2 注:CaCl2含量占比固定为4%,基础盐系即MgCl2+NaCl的含量占比由1 - FeCl2% - FeCl3% - CaCl2%得出 表 3 氯化熔盐在750 ℃条件下的电导率分析结果
Table 3. Conductivity analysis results of chlorinated molten salt at 750 ℃
试验号 w(FeCl2)/% w(FeCl3)/% 基础盐系L κ/(S·cm−1) 1 5 0 3:2 1.30 2 5 5 1:1 1.45 3 5 10 2:3 1.42 4 5 15 1:2 1.18 5 10 0 1:1 1.58 6 10 5 3:2 1.17 7 10 10 1:2 2.35 8 10 15 2:3 1.30 9 15 0 2:3 1.29 10 15 5 1:2 2.24 11 15 10 3:2 1.22 12 15 15 1:1 2.84 13 20 0 1:2 1.45 14 20 5 2:3 2.99 15 20 10 1:1 1.63 16 20 15 3:2 3.23 k1 1.337 1.405 1.730 k2 1.600 1.963 1.875 k3 1.897 1.655 1.750 k4 2.325 2.138 1.805 极差R 0.988 0.733 0.145 表 4 氯化熔盐在750 ℃条件下的粘度分析结果
Table 4. Viscosity analysis results of chlorinated molten salt at 750 ℃
试验号 w(FeCl2)/% w(FeCl3)/% 基础盐系L η/(mPa·s) 1 5 0 3:2 4.69 2 5 5 1:1 4.89 3 5 10 2:3 5.28 4 5 15 1:2 6.36 5 10 0 1:1 5.24 6 10 5 3:2 5.49 7 10 10 1:2 5.10 8 10 15 2:3 6.38 9 15 0 2:3 5.77 10 15 5 1:2 4.97 11 15 10 3:2 5.77 12 15 15 1:1 5.49 13 20 0 1:2 5.01 14 20 5 2:3 5.21 15 20 10 1:1 5.40 16 20 15 3:2 5.78 k1 5.305 5.178 5.433 k2 5.553 5.140 5.255 k3 5.500 5.387 5.660 k4 5.350 6.003 5.360 极差R 0.248 0.863 0.405 表 5 氯化熔盐在750 ℃条件下的多指标综合评分结果
Table 5. Results of multi-index comprehensive score of chlorinated molten salt at 750 ℃
试验号 w(FeCl2)/% w(FeCl3)/% 基础盐系L 电导率
隶属度粘度
隶属度综合分 1 5 0 3:2 0.06 1.00 0.53 2 5 5 1:1 0.14 0.88 0.51 3 5 10 2:3 0.12 0.65 0.39 4 5 15 1:2 0.00 0.01 0.01 5 10 0 1:1 0.20 0.67 0.44 6 10 5 3:2 0.00 0.53 0.26 7 10 10 1:2 0.57 0.76 0.67 8 10 15 2:3 0.06 0.00 0.03 9 15 0 2:3 0.06 0.36 0.21 10 15 5 1:2 0.52 0.83 0.68 11 15 10 3:2 0.02 0.36 0.19 12 15 15 1:1 0.81 0.53 0.67 13 20 0 1:2 0.14 0.81 0.47 14 20 5 2:3 0.88 0.69 0.79 15 20 10 1:1 0.22 0.58 0.40 16 20 15 3:2 1.00 0.36 0.68 k1 1.44 1.65 1.66 k2 1.40 2.24 2.02 k3 1.75 1.65 1.42 k4 2.34 1.39 1.83 极差R 0.94 0.85 0.60 -
[1] Yang Fang, Li Yanli, Shen Chengxiu, et al. Research progress on preparation and forming technology of titanium and titanium alloy powder[J]. Powder Metallurgy Technology, 2023,41(4):330-337. (杨芳, 李延丽, 申承秀, 等. 钛及钛合金粉末制备与成形工艺研究进展[J]. 粉末冶金技术, 2023,41(4):330-337.Yang Fang, Li Yanli, Shen Chengxiu, et al. Research progress on preparation and forming technology of titanium and titanium alloy powder[J]. Powder Metallurgy Technology, 2023, 41(4): 330-337. [2] Isaac M M, Mxolisi B S. Effects of porosity on the corrosion behaviour of PM-fabricated titanium foams for biomedical applications[J]. International Journal of Electrochemical Science, 2024,19(3):100495. doi: 10.1016/j.ijoes.2024.100495 [3] Yang Yaohui, Hui Bo, Yan Shiqiang, et al. Research progress on global vanadium-titanium-magnetite resources and comprehensive utilization[J]. Multipurpose Utilization of Mineral Resources, 2023(4):1-11. (杨耀辉, 惠博, 颜世强, 等. 全球钒钛磁铁矿资源概况与综合利用研究进展[J]. 矿产综合利用, 2023(4):1-11. doi: 10.3969/j.issn.1000-6532.2023.04.001Yang Yaohui, Hui Bo, Yan Shiqiang, et al. Research progress on global vanadium-titanium-magnetite resources and comprehensive utilization[J]. Multipurpose Utilization of Mineral Resources, 2023(4): 1-11. doi: 10.3969/j.issn.1000-6532.2023.04.001 [4] Fu Ganghua, Yao Hongguo, Chen Feng, et al. Research progress on comprehensive utilization of chlorination waste slag of molten salt[J]. Multipurpose Utilization of Mineral Resources, 2023(3):112-118. (付刚华, 姚洪国, 陈凤, 等. 熔盐氯化废渣综合利用研究进展[J]. 矿产综合利用, 2023(3):112-118. doi: 10.3969/j.issn.1000-6532.2023.03.019Fu Ganghua, Yao Hongguo, Chen Feng, et al. Research progress on comprehensive utilization of chlorination waste slag of molten salt[J]. Multipurpose Utilization of Mineral Resources, 2023(3): 112-118. doi: 10.3969/j.issn.1000-6532.2023.03.019 [5] Li Liang. Research progress on the application and technology of titanium tetrachloride at home and abroad[J]. Light Metals, 2021(10):42-48. (李亮. 国内外四氯化钛的应用及工艺技术研究进展[J]. 轻金属, 2021(10):42-48.Li Liang. Research progress on the application and technology of titanium tetrachloride at home and abroad[J]. Light Metals, 2021(10): 42-48. [6] Luo Zaiguo, Yang Zhen, Yang Xiaodong, et al. Study on the production of TiCl4 by boiling chlorination furnace without sieve plate[J]. Yunnan Metallurgy, 2018,47(2):65-68. (罗在国, 杨振, 杨晓东, 等. 无筛板沸腾氯化炉生产TiCl4工艺研究[J]. 云南冶金, 2018,47(2):65-68. doi: 10.3969/j.issn.1006-0308.2018.02.010Luo Zaiguo, Yang Zhen, Yang Xiaodong, et al. Study on the production of TiCl4 by boiling chlorination furnace without sieve plate[J]. Yunnan Metallurgy, 2018, 47(2): 65-68. doi: 10.3969/j.issn.1006-0308.2018.02.010 [7] Wang Jun, Zhao Yingtao, Cao Li, et al. Numerical simulation of boiling chlorinated gas-solid two-phase flow in a spouted bed of titanium slag[J]. Conservation and Utilization of Mineral Resources, 2017(6):66-74. (王军, 赵英涛, 曹丽, 等. 钛渣喷动床沸腾氯化气固两相流数值模拟[J]. 矿产保护与利用, 2017(6):66-74.Wang Jun, Zhao Yingtao, Cao Li, et al. Numerical simulation of boiling chlorinated gas-solid two-phase flow in a spouted bed of titanium slag[J]. Conservation and Utilization of Mineral Resources, 2017(6): 66-74. [8] Bordbar H, Yousefi A A, Abedini H. Production of titanium tetrachloride (TiCl4) from titanium ores: A review[J]. Polyolefins Journal, 2017,4(2):149-173. [9] Kroll W. The production of ductile titanium[J]. Journal of the Electrochemical Society, 1940,78(1):35-47. [10] Qi Manfu. Analysis of titanium tetrachloride production technology[J]. Chemical Enterprise Management, 2022(3):55-57. (齐满富. 四氯化钛生产工艺分析[J]. 化工管理, 2022(3):55-57.Qi Manfu. Analysis of titanium tetrachloride production technology[J]. Chemical Enterprise Management, 2022(3): 55-57. [11] Yang Xinping, Wang Xiufeng. Research progress in conductivity measurement of high-temperature melt[J]. China Ceramics, 2010,46(11):12-16. (杨新平, 王秀峰. 高温熔体电导率测试研究进展[J]. 中国陶瓷, 2010,46(11):12-16.Yang Xinping, Wang Xiufeng. Research progress in conductivity measurement of high-temperature melt[J]. China Ceramics, 2010, 46(11): 12-16. [12] Kan H M, Wang Z W, Ban Y G, et al. Electrical conductivity of Na3AlF6-AlF3-Al2O3-CaF2-LiF(NaCl) system electrolyte[J]. Transactions of Nonferrous Metals Society of China, 2007(1):181-186. [13] Shigeta H, Hidehiro H, Kazumi O. Electrical conductivity of molten slags for electro-slag remelting[J]. Transactions of the Iron & Steel Institute of Japan, 2006,23(12):1053-1058. [14] Long Yao, Yu Zhefeng, Wang Xin, et al. Research progress of viscosity and measurement technology of high-temperature melt[J]. Materials Research and Application, 2023,17(3):483-494. (龙耀, 于哲峰, 王昕, 等. 高温熔体粘度及其测量技术的研究进展[J]. 材料研究与应用, 2023,17(3):483-494.Long Yao, Yu Zhefeng, Wang Xin, et al. Research progress of viscosity and measurement technology of high-temperature melt[J]. Materials Research and Application, 2023, 17(3): 483-494. [15] Shao Hongfei, Liu Yuanjun, Ren Wanjie, et al. Research progress of viscosity measurement methods and reference materials for non-Newtonian fluids[J]. Journal of Astronautic Metrology and Measurement, 2019,39(Z1):1-5. (邵鸿飞, 刘元俊, 任万杰, 等. 非牛顿流体粘度测试方法及标准物质研究进展[J]. 宇航计测技术, 2019,39(Z1):1-5.Shao Hongfei, Liu Yuanjun, Ren Wanjie, et al. Research progress of viscosity measurement methods and reference materials for non-Newtonian fluids[J]. Journal of Astronautic Metrology and Measurement, 2019, 39(Z1): 1-5. [16] Wang Xiaojie, Zhu Shanshan, Wang Xiaopo, et al. High pressure liquid viscosity test system for falling body method[J]. Journal of Engineering Thermophysics, 2020,41(4):788-791. (王小杰, 朱山杉, 王晓坡, 等. 落体法流体高压液相黏度实验系统[J]. 工程热物理学报, 2020,41(4):788-791.Wang Xiaojie, Zhu Shanshan, Wang Xiaopo, et al. High pressure liquid viscosity test system for falling body method[J]. Journal of Engineering Thermophysics, 2020, 41(4): 788-791. [17] Wei Xiaolan, Xie Pei, Wang Weilong, et al. Calculation of phase diagram of ternary chloride system containing calcium and thermal stability of molten salt[J]. CIESC Journal, 2021,72(6):3074-3083. (魏小兰, 谢佩, 王维龙, 等. 含钙三元氯化物体系相图计算与熔盐热稳定性[J]. 化工学报, 2021,72(6):3074-3083.Wei Xiaolan, Xie Pei, Wang Weilong, et al. Calculation of phase diagram of ternary chloride system containing calcium and thermal stability of molten salt[J]. CIESC Journal, 2021, 72(6): 3074-3083. [18] Yin Yue. Thermal stability of chloride molten salt and thermal properties of molten salt enhancement[D]. Guangzhou: South China University of Technology, 2018. (尹月. 氯化物熔盐热稳定性与熔盐热物性强化[D]. 广州: 华南理工大学, 2018.Yin Yue. Thermal stability of chloride molten salt and thermal properties of molten salt enhancement[D]. Guangzhou: South China University of Technology, 2018. [19] Wu J, Ni H, Liang W, et al. Molecular dynamics simulation on local structure and thermodynamic properties of molten ternary chlorides systems for thermal energy storage[J]. Computational Materials Science, 2019,170:109051. doi: 10.1016/j.commatsci.2019.05.049 [20] Chen Feng, Wen Yuekai, Guo Yufeng, et al. Research status of viscosity characteristics of chlorinated molten salt system[J]. Inorganic Chemicals Industry, 2022,54(6):1-5. (陈凤, 问悦凯, 郭宇峰, 等. 氯化熔盐体系黏度特性研究现状[J]. 无机盐工业, 2022,54(6):1-5.Chen Feng, Wen Yuekai, Guo Yufeng, et al. Research status of viscosity characteristics of chlorinated molten salt system[J]. Inorganic Chemicals Industry, 2022, 54(6): 1-5. [21] Fan Jianfeng, Yuan Zhangfu, Li Jing, et al. Viscosity of molten CaCl2-MgCl2 system[J]. The Chinses Journal of Nonferrous Metals, 2004(10):1759-1762. (范建峰, 袁章福, 李晶, 等. 熔融CaCl2-MgCl2体系的粘度[J]. 中国有色金属学报, 2004(10):1759-1762. doi: 10.3321/j.issn:1004-0609.2004.10.024Fan Jianfeng, Yuan Zhangfu, Li Jing, et al. Viscosity of molten CaCl2-MgCl2 system[J]. The Chinses Journal of Nonferrous Metals, 2004(10): 1759-1762. doi: 10.3321/j.issn:1004-0609.2004.10.024 [22] Wei Xiaolan, Xie Pei, Zhang Xuechuan, et al. Study on preparation and thermophysical properties of chloride molten salt materials[J]. CIESC Journal, 2020,71(5):2423-2431. (魏小兰, 谢佩, 张雪钏, 等. 氯化物熔盐材料的制备及其热物理性质研究[J]. 化工学报, 2020,71(5):2423-2431.Wei Xiaolan, Xie Pei, Zhang Xuechuan, et al. Study on preparation and thermophysical properties of chloride molten salt materials[J]. CIESC Journal, 2020, 71(5): 2423-2431. -





 下载:
下载: