Thermodynamic model of dephosphorization of CaO-SiO2-MgO-Al2O3-FeO-P2O5-TiO2 slag system based on IMCT theory
-
摘要: 基于离子分子共存理论(IMCT)建立了CaO-SiO2-MgO-Al2O3-FeO-P2O5-TiO2七元熔渣磷分配比(LP)模型,该模型已在多个熔渣体系中被验证,具有较为精确的预测磷富集行为的能力,进一步分析了各组元成分对活度及LP的影响,通过该模型总结了冶炼钒钛磁铁矿的合理熔渣成分。结果表明:在
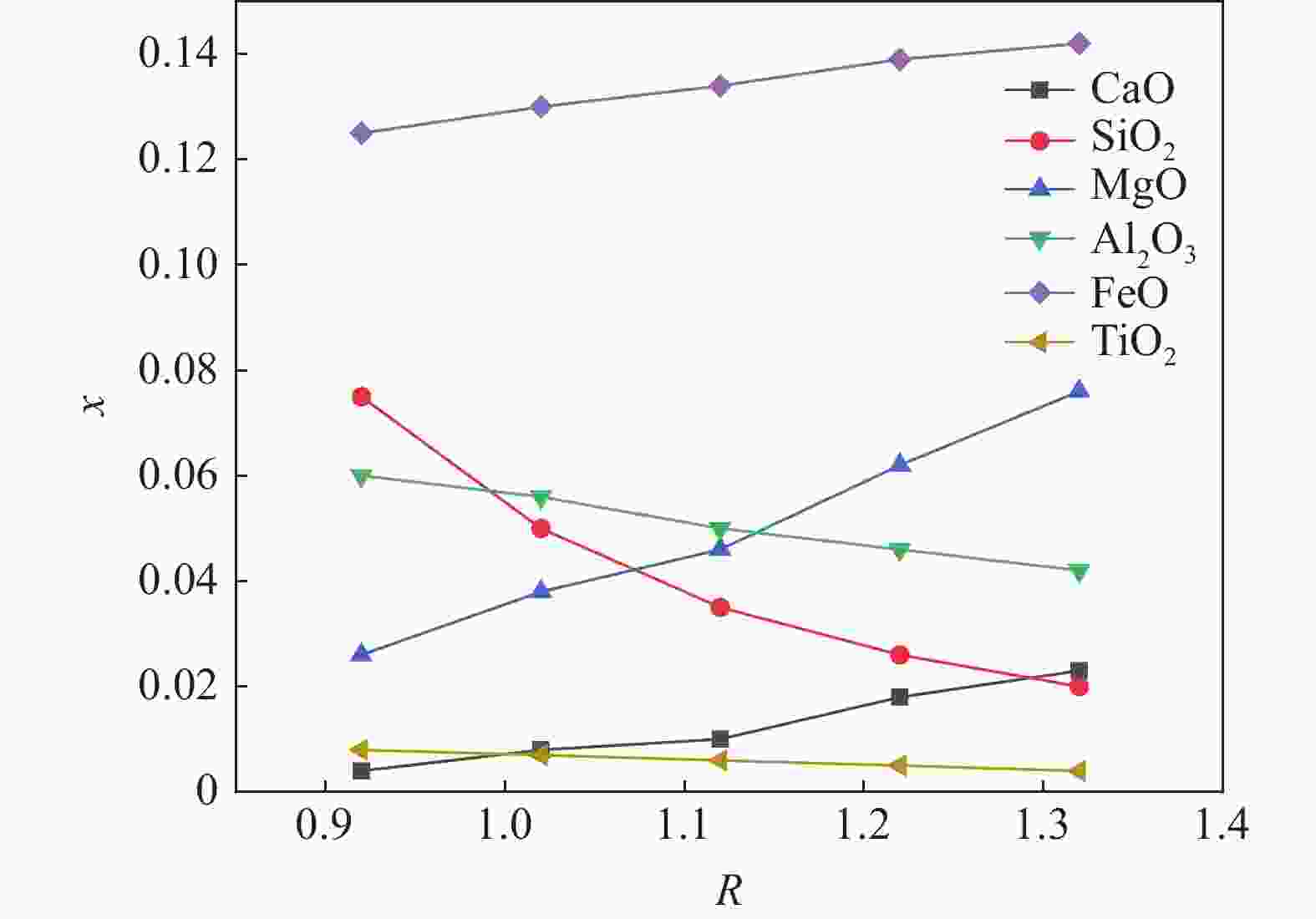
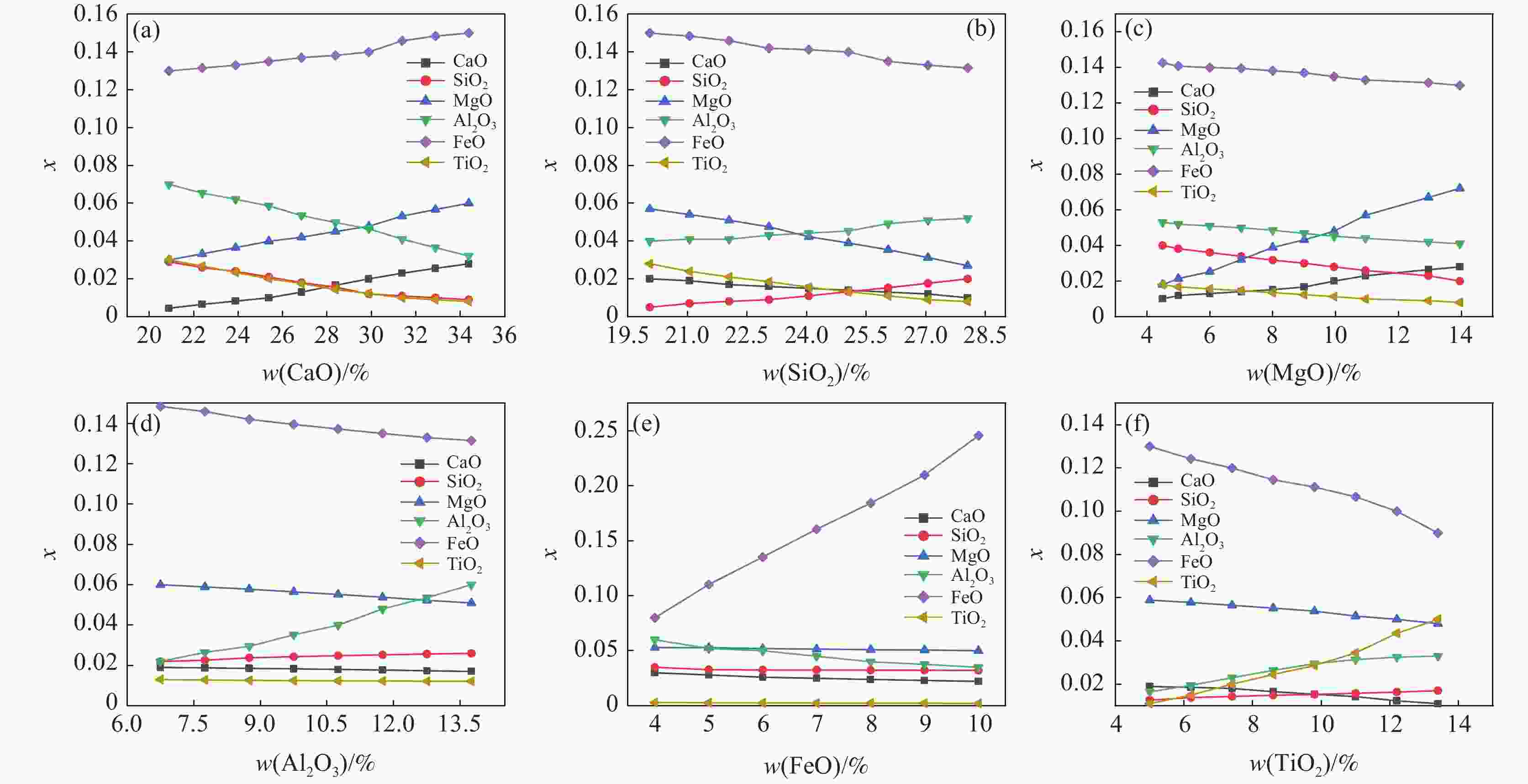
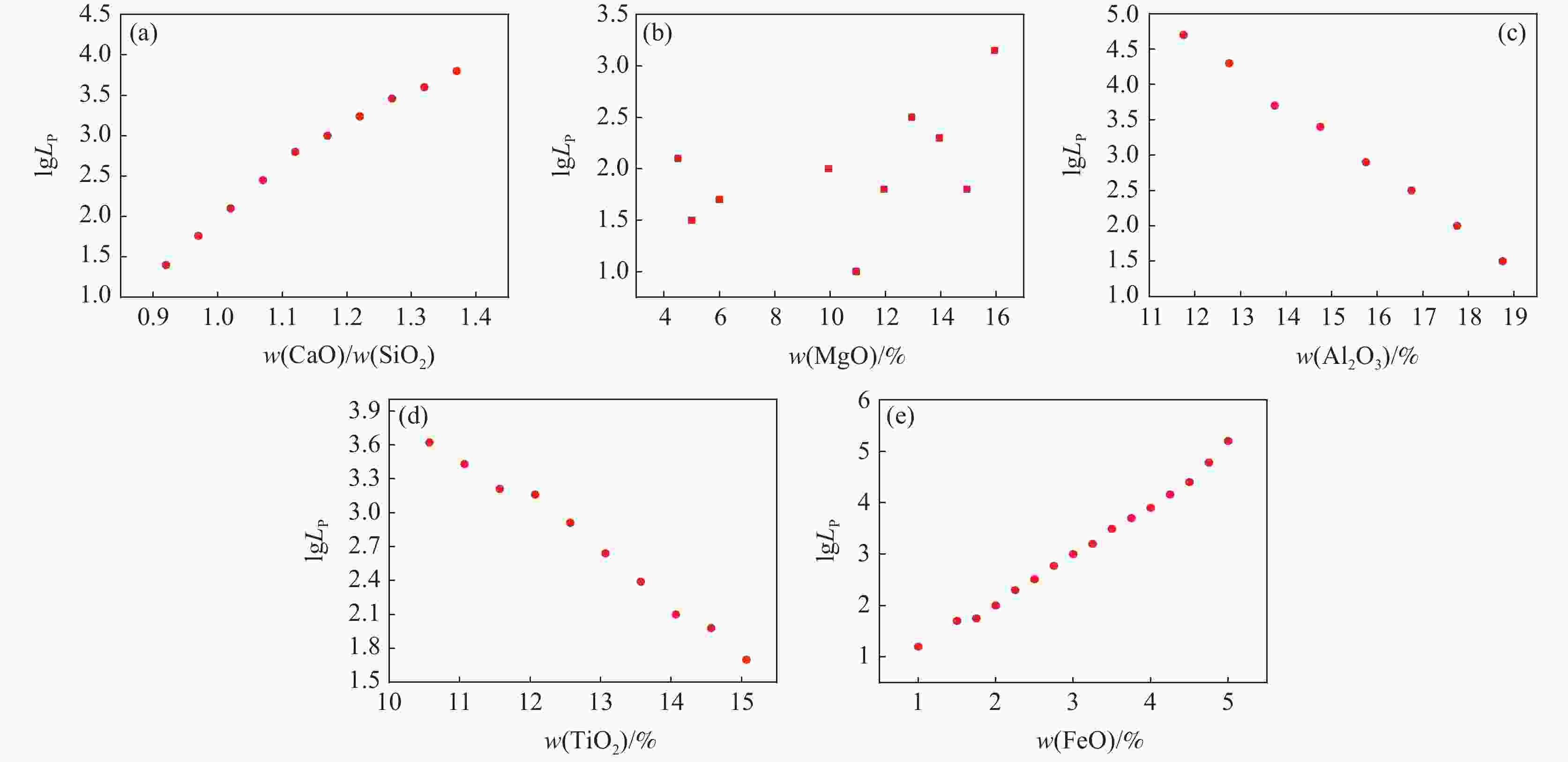
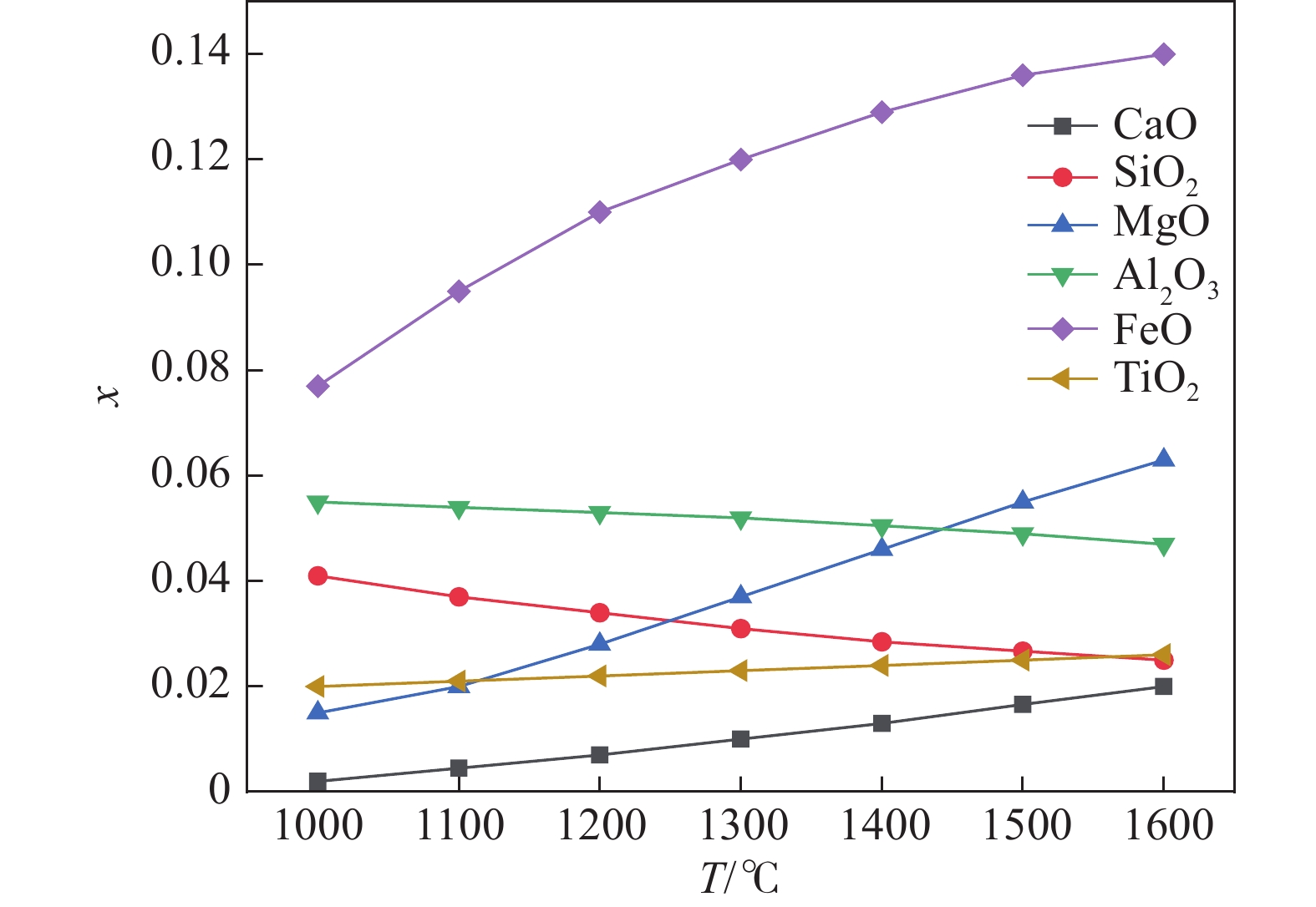
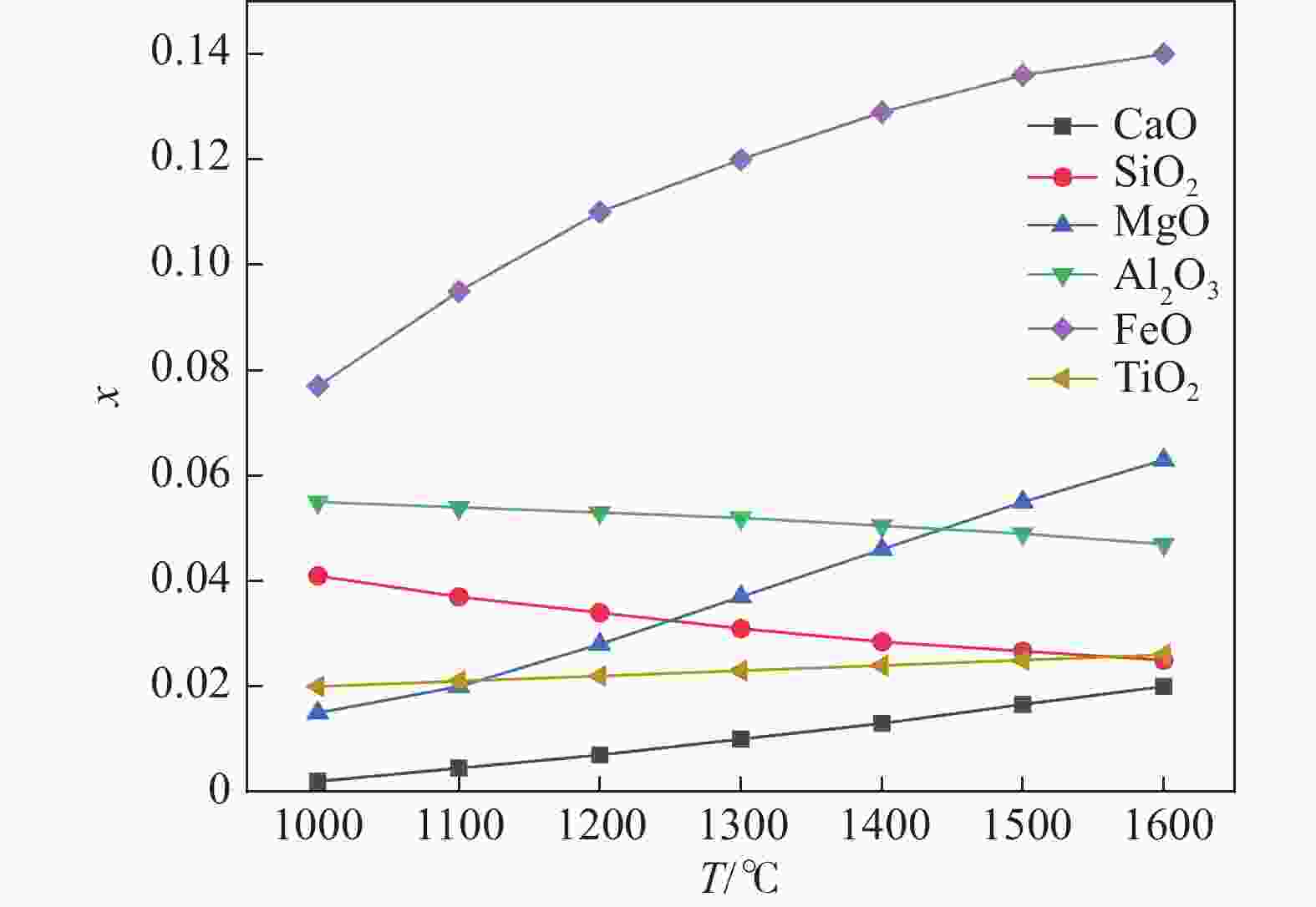
1000 ~1600 ℃范围内,随着温度的升高,FeO、MgO与CaO活度上升,SiO2与Al2O3的活度随之减小,对TiO2无明显影响。随着碱度由0.92升高至1.32,CaO、MgO的活度明显上升,SiO2和Al2O3的活度明显下降,FeO的活度逐渐增加,TiO2的活度基本保持不变。随着渣中CaO质量分数增加,Al2O3、SiO2的活度随之减少,CaO、MgO、FeO的活度随之增大。随着渣中SiO2质量分数增加,渣中碱性氧化物CaO、MgO、FeO的活度随之减少,渣中酸性氧化物SiO2、Al2O3、SiO2的活度随之增加。渣系中MgO质量分数由4%增加到14.5%后,各组元活度的变化规律与CaO基本相同,但影响程度弱于CaO。渣系中Al2O3、FeO和TiO2质量分数增加后,仅使得自身活度显著增加,对其它组元的活度影响程度相对较小;随着碱度和FeO质量分数的增加,LP逐渐增加;随着MgO质量分数的增加,Lp先降低后增加;随Al2O3、TiO2质量分数的增加,LP逐渐降低;TiO2质量分数在10%左右时,选取熔渣组分为CaO(35.5%)-SiO2(26%)-MgO(10.2%)-Al2O3(12.5%)-FeO(5%)- TiO2,铁水中[P]可控制在0.01%以下。Abstract: Based on the theory of ionic-molecular coexistence (IMCT), a seven-component slag phosphorus distribution ratio (LP) model for CaO-SiO2-MgO-Al2O3-FeO-P2O5-TiO2 was established. This model has been validated in multiple slag systems and has the ability to accurately predict the enrichment behavior of phosphorus. The influence of each component on activity and LP was further analyzed, and the reasonable slag composition for smelting vanadium-titanium magnetite was summarized through this model. The results show that within the temperature range of1000 to1600 °C, as the temperature increases, the activities of FeO, MgO and CaO increase, while those of SiO2 and Al2O3 decrease, with no significant effect on TiO2. As the basicity increases from 0.92 to 1.32, the activities of CaO and MgO significantly increase, while those of SiO2 and Al2O3 decrease significantly, with a gradual increase in the activity of FeO and a nearly constant activity of TiO2. As the mass fraction of CaO in the slag increases, the activities of Al2O3 and SiO2 decrease, while those of CaO, MgO and FeO increase. As the mass fraction of SiO2 in the slag increases, the activities of basic oxides CaO, MgO and FeO decrease, while those of acidic oxides SiO2, Al2O3 and TiO2 increase. After increasing the mass fraction of MgO in the slag from 4% to 14.5%, the variation law of each component activity is basically the same as that of CaO, but the influence is weaker than that of CaO. After increasing the mass fraction of Al2O3, FeO and TiO2 in the slag, only their own activities significantly increase, with relatively small effects on the activities of other components. As the basicity and FeO mass fraction increase, LP gradually increases. As the MgO mass fraction increases, Lp first decreases and then increases. As the Al2O3 and TiO2 mass fractions increase, LP gradually decreases. When the TiO2 mass fraction is around 10%, the slag composition of CaO (35.5%)-SiO2 (26%)-MgO (10.2%)-Al2O3 (12.5%)-FeO (5%)-TiO2 is selected, and the [P] in the molten iron can be controlled below 0.01%. -
表 1 炉渣界面脱磷反应的标准吉布斯自由能和自由常数
Table 1. Standard Gibbs free energy and free constant of slag interface dephosphorization reaction
炉渣界面脱磷反应 $ \Delta_{\text{r}}G_{^{\text{m}}}^{\mathrm{\theta}}/\left(\text{J}\cdot\text{mo}\text{l}^{-1}\right) $ $ K_{\text{c}i}^{\theta} $ $ {\text{5(Fe}}_{\text{t}}^{{\text{2}}+} + {{\text{O}}^{{\text{2}} - }}{\text{)}} + {\text{2}}\left[ {\text{P}} \right] = {{{\mathrm{P}}_2 {\mathrm{O}}_5}} + {\text{5tFe}} $ $ - 122\;412 + 312.522 T $ $ K_{{{{\mathrm{P}}_2 {\mathrm{O}}_5}}}^{\theta} = \dfrac{{{N_{{\mathrm{P}}_2 {\mathrm{O}}_5}}a_{{\text{Fe}}}^{5{\text{t}}}}}{{N_{\rm{{Fe_t}O}}^5 a_{\left[ {\text{P}} \right]}^2}} $ $ {\text{2(C}}{{\text{a}}^{{\text{2}} + }} + {{\text{O}}^{{\text{2}} - }}{\text{)}} + {\text{5(Fe}}_{\text{t}}^{{\text{2}} + } + {{\text{O}}^{{\text{2}} - }}{\text{)}} + {\text{2}}\left[ {\text{P}} \right] = \left( {{\text{2CaO}} \cdot {\mathrm{P}}_2 {\mathrm{O}}_5} \right) + {\text{5tFe}} $ $ - 680\;599 + 330.552 T $ $ K_{2\text{CaO}\cdot\mathrm{P}_2\mathrm{O}_5}^{\theta}=\dfrac{N_{2\text{CaO}\cdot\mathrm{P}_2\mathrm{O}_5}a_{\text{Fe}}^{5\text{t}}}{N_{\text{CaO}}^2N_{{{\mathrm{Fe}}_{\mathrm{t}}{\mathrm{O}}}}^5a_{\left[\text{P}\right]}^2} $ $ {\text{3(C}}{{\text{a}}^{{\text{2}} + }} + {{\text{O}}^{{\text{2}} - }}{\text{)}} + {\text{5(Fe}}_{\text{t}}^{{\text{2}} + } + {{\text{O}}^{{\text{2}} - }}{\text{)}} + {\text{2}}\left[ {\text{P}} \right] = \left( {{\text{3CaO}} \cdot {\mathrm{P}}_2 {\mathrm{O}}_5} \right) + {\text{5tFe}} $ $ - 805\;282 + 301.264 T $ $ K_{3{\text{CaO}} \cdot {{{\mathrm{P}}_2 {\mathrm{O}}}}_5}^{\theta} = \dfrac{{{N_{3{\text{CaO}} \cdot {{{\mathrm{P}}_2 {\mathrm{O}}}}_5}}a_{{\text{Fe}}}^{5{\text{t}}}}}{{N_{{\text{CaO}}}^3 N_{\rm{{Fe_t}O}}^5 a_{\left[ {\text{P}} \right]}^2}} $ $ {\text{4(C}}{{\text{a}}^{{\text{2}} + }} + {{\text{O}}^{{\text{2}} - }}{\text{)}} + {\text{5(Fe}}_{\text{t}}^{{\text{2}} + } + {{\text{O}}^{{\text{2}} - }}{\text{)}} + {\text{2}}\left[ {\text{P}} \right] = \left( {{\text{4CaO}} \cdot {\mathrm{P}}_2 {\mathrm{O}}_5} \right) + {\text{5tFe}} $ $ - 565\;964 + 291.641 T $ $ K_{4{\text{CaO}} \cdot {{{\mathrm{P}}_2 {\mathrm{O}}}}_5}^{\theta} = \dfrac{{{N_{4{\text{CaO}} \cdot {{{\mathrm{P}}_2 {\mathrm{O}}}}_5}}a_{{\text{Fe}}}^{5{\text{t}}}}}{{N_{{\text{CaO}}}^4 N_{\rm{{Fe_t}O}}^5 a_{\left[ {\text{P}} \right]}^2}} $ $ {\text{2(M}}{{\text{g}}^{{\text{2}} + }} + {{\text{O}}^{{\text{2}} - }}{\text{)}} + {\text{5(Fe}}_{\text{t}}^{{\text{2}} + } + {{\text{O}}^{{\text{2}} - }}{\text{)}} + {\text{2}}\left[ {\text{P}} \right] = \left( {{\text{2MgO}} \cdot {\mathrm{P}}_2 {\mathrm{O}}_5} \right) + {\text{5tFe}} $ $ 72\;977 - 44.243 T $ $ K_{2{\text{MgO}} \cdot {{{\mathrm{P}}_2 {\mathrm{O}}}}_5}^{\theta} = \dfrac{{{N_{2{\text{MgO}} \cdot {{{\mathrm{P}}_2 {\mathrm{O}}}}_5}}a_{{\text{Fe}}}^{5{\text{t}}}}}{{N_{{\text{MgO}}}^2 N_{\rm{{Fe_t}O}}^5 a_{\left[ {\text{P}} \right]}^2}} $ $ {\text{3(M}}{{\text{g}}^{{\text{2}} + }} + {{\text{O}}^{{\text{2}} - }}{\text{)}} + {\text{5(Fe}}_{\text{t}}^{{\text{2}} + } + {{\text{O}}^{{\text{2}} - }}{\text{)}} + {\text{2}}\left[ {\text{P}} \right] = \left( {{\text{3MgO}} \cdot {\mathrm{P}}_2 {\mathrm{O}}_5} \right) + {\text{5tFe}} $ $ - 484\;369 + 254.831 T $ $ K_{3{\text{MgO}} \cdot {{{\mathrm{P}}_2 {\mathrm{O}}}}_5}^{\theta} = \dfrac{{{N_{3{\text{MgO}} \cdot {{{\mathrm{P}}_2 {\mathrm{O}}}}_5}}a_{{\text{Fe}}}^{5{\text{t}}}}}{{N_{{\text{MgO}}}^3 N_{\rm{{Fe_t}O}}^5 a_{\left[ {\text{P}} \right]}^2}} $ $ {\text{5(Fe}}_{\text{t}}^{{\text{2}} + } + {{\text{O}}^{{\text{2}} - }}{\text{)}} + {\text{3(F}}{{\text{e}}^{{\text{2}} + }} + {{\text{O}}^{{\text{2}} - }}{\text{)}} + {\text{2}}\left[ {\text{P}} \right] = \left( {{\text{3FeO}} \cdot {\mathrm{P}}_2 {\mathrm{O}}_5} \right) + {\text{5tFe}} $ $ - 525\;769 + 387.822 T $ $ K_{3\text{FeO}\cdot\mathrm{P}_2\mathrm{O}_5}^{{\theta}}=\dfrac{N_{3\text{FeO}\cdot\mathrm{P}_2\mathrm{O}_5}a_{\text{Fe}}^{5\text{t}}}{N_{\mathrm{Fe}_{\mathrm{t}}\mathrm{O}}^5N_{\text{FeO}}^3a_{\left[\text{P}\right]}^2} $ $ {\text{(Fe}}_{\text{t}}^{{\text{2}} + } + {{\text{O}}^{{\text{2}} - }}{\text{)}} + {\text{4(F}}{{\text{e}}^{{\text{2}} + }} + {{\text{O}}^{{\text{2}} - }}{\text{)}} + {\text{2}}\left[ {\text{P}} \right] = \left( {{\text{4FeO}} \cdot {\mathrm{P}}_2 {\mathrm{O}}_5} \right) + {\text{5tFe}} $ $ - 477\;223 + 342.481 T $ $ K_{4{\text{FeO}} \cdot {{{\mathrm{P}}_2 {\mathrm{O}}}}_5}^{\theta} = \dfrac{{{N_{4{\text{FeO}} \cdot {{{\mathrm{P}}_2 {\mathrm{O}}}}_5}}a_{{\text{Fe}}}^{5{\text{t}}}}}{{N_{\rm{{Fe_t}O}}^5 N_{{\text{FeO}}}^4 a_{\left[ {\text{P}} \right]}^2}} $ 表 2 高磷钒钛矿炉渣最佳炉渣成分计算
Table 2. The optimal slag compositions of high phosphorus vanadium titanium ore slag
% TiO2 CaO SiO2 MgO Al2O3 FeO 模型预测 10.5 35.5 26 10.2 12.5 5 60%钒钛磁铁矿[6] 9.92 33.26 26.6 7.43 12.97 6.85 -
[1] Chen Weibin, Dong Zhaoqi, Jiao Yang, et al. Preparation, sintering behavior and consolidation mechanism of vanadium-titanium magnetite pellets[J]. Crystals, 2021,11(2):188. doi: 10.3390/cryst11020188 [2] Lü Jiawei, Yu Zexin, Li Jiulong, et al. Characteristics of vanadium-titanium magnetite resources in Chaoyang, Liaoning and their development and utilization prospects[J]. Nonferrous Mining and Metallurgy, 2023,39(3):9-11. (吕佳卫, 于泽新, 李玖龙, 等. 辽宁朝阳钒钛磁铁矿资源特点及开发利用前景[J]. 有色矿冶, 2023,39(3):9-11. doi: 10.3969/j.issn.1007-967X.2023.03.003Lü Jiawei, Yu Zexin, Li Jiulong, et al. Characteristics of vanadium-titanium magnetite resources in Chaoyang, Liaoning and their development and utilization prospects[J]. Nonferrous Mining and Metallurgy, 2023, 39(3): 9-11. doi: 10.3969/j.issn.1007-967X.2023.03.003 [3] Wang Yang, Yang Shufeng, Li Jingshe, et al. Dephosphorization by double-slag process in converter steelmaking[J]. High Temperature Materials and Processes, 2018,37(7):625-633. [4] Li Chenxiao, Xue Yuekai, Wang Shuhuan, et al. Development and application of converter slag gasification for phosphorus removal in cyclic steelmaking technology[J]. Iron and Steel, 2023,58(7):64-71, 143. (李晨晓, 薛月凯, 王书桓, 等. 转炉熔渣气化脱磷循环炼钢技术开发及应用[J]. 钢铁, 2023,58(7):64-71, 143.Li Chenxiao, Xue Yuekai, Wang Shuhuan, et al. Development and application of converter slag gasification for phosphorus removal in cyclic steelmaking technology[J]. Iron and Steel, 2023, 58(7): 64-71, 143. [5] Wu Yaoting, Zhang Yanling, Zhao Zheng. Influence of CaO-SiO2-Fe2O3-Al2O3-Na2O slag system on simultaneous desulfurization and dephosphorization behavior of molten iron[J]. Journal of Iron and Steel Research, 2023,35(9):1074-1083. (吴耀庭, 张延玲, 赵峥. CaO-SiO2-Fe2O3-Al2O3-Na2O渣系对铁水同时脱磷脱硫行为的影响[J]. 钢铁研究学报, 2023,35(9):1074-1083.Wu Yaoting, Zhang Yanling, Zhao Zheng. Influence of CaO-SiO2-Fe2O3-Al2O3-Na2O slag system on simultaneous desulfurization and dephosphorization behavior of molten iron[J]. Journal of Iron and Steel Research, 2023, 35(9): 1074-1083. [6] Liu Jun, Peng Zhonghui. Industrial experiment of vanadium-titanium magnetite smelting using the HIsmelt process[J]. Ironmaking, 2022,41(1):57-61. (刘军, 彭忠辉. HIsmelt工艺冶炼钒钛磁铁矿工业试验[J]. 炼铁, 2022,41(1):57-61.Liu Jun, Peng Zhonghui. Industrial experiment of vanadium-titanium magnetite smelting using the HIsmelt process[J]. Ironmaking, 2022, 41(1): 57-61. [7] Lan Chenchen, Gao Yanjia, Lü Qing, et al. Current status and prospects of smelting reduction ironmaking technology in China[J]. Comprehensive Utilization of Mineral Resources, 2022(11):1-15. (兰臣臣, 高艳甲, 吕庆, 等. 我国熔融还原炼铁技术发展现状及展望[J]. 矿产综合利用, 2022(11):1-15.Lan Chenchen, Gao Yanjia, Lü Qing, et al. Current status and prospects of smelting reduction ironmaking technology in China[J]. Comprehensive Utilization of Mineral Resources, 2022(11): 1-15. [8] Yan Guangshi, Liu Ran, Lan Chenchen, et al. Development and prospects of the HIsmelt smelting reduction process[J]. Hebei Metallurgy, 2023(8):7-12. (闫光石, 刘然, 兰臣臣, 等. HIsmelt熔融还原工艺的发展及展望[J]. 河北冶金, 2023(8):7-12.Yan Guangshi, Liu Ran, Lan Chenchen, et al. Development and prospects of the HIsmelt smelting reduction process[J]. Hebei Metallurgy, 2023(8): 7-12. [9] Zhang Xiangguo, Jia Lijun. Current development and production practice of smelting reduction ironmaking technology in China[J]. Metallurgy and Materials, 2019,39(4):90-91. (张向国, 贾利军. 我国熔融还原炼铁技术发展现状及生产实践[J]. 冶金与材料, 2019,39(4):90-91. doi: 10.3969/j.issn.1674-5183.2019.04.048Zhang Xiangguo, Jia Lijun. Current development and production practice of smelting reduction ironmaking technology in China[J]. Metallurgy and Materials, 2019, 39(4): 90-91. doi: 10.3969/j.issn.1674-5183.2019.04.048 [10] Wu Longfei, Yang Guangqing, Ma Baoliang. Development history and improvement direction of the HIsmelt smelting reduction process[J]. Hebei Metallurgy, 2021(9):8-10, 61. (武龙飞, 杨广庆, 马保良. HIsmelt熔融还原工艺的发展历程及改进方向[J]. 河北冶金, 2021(9):8-10, 61.Wu Longfei, Yang Guangqing, Ma Baoliang. Development history and improvement direction of the HIsmelt smelting reduction process[J]. Hebei Metallurgy, 2021(9): 8-10, 61. [11] Yang X M, Zhang M, Chai G M, et al. Thermodynamic models for predicting dephosphorisation ability and potential of CaO–FeO–Fe2O3–Al2O3–P2O5 slags during secondary refining process of molten steel based on ion and molecule coexistence theory[J]. Ironmaking & Steelmaking, 2016,43(9):663-687. [12] Zheng Xiang, Liu Chengjun. Thermodynamic properties assessment of CaO-Al2O3-Ce2O3 system[J]. Metallurgical and Materials Transactions B, 2021,52(5):3183-3192. [13] Li Pengcheng, Zhang Jianliang. Retraction: A prediction model of phosphorus distribution between CaO–SiO2–MgO–FeO–Fe2O3–P2O5 slags and liquid iron[J]. ISIJ International, 2014,54(4):756-765. doi: 10.2355/isijinternational.54.756 [14] Duan S C, Guo X L, Guo H J, et al. A manganese distribution prediction model for CaO–SiO2–FeO–MgO–MnO–Al2O3 slags based on IMCT[J]. Ironmaking & Steelmaking, 2016,44(3):168-184. [15] Duan Shengchao, Li Chuang, Guo Xiaolong, et al. A thermodynamic model for calculating manganese distribution ratio between CaO–SiO2–MgO–FeO–MnO–Al2O3–TiO2–CaF2 ironmaking slags and carbon saturated hot metal based on the IMCT[J]. Ironmaking & Steelmaking, 2017,45(7):655-664. [16] Sun Han, Yang Jian, Yang Wenkui, et al. Evaluation of phosphorus enrichment capacity of CaO–SiO2–FeO–MgO–MnO–P2O5–Al2O3 dephosphorization slag based on ion-molecule coexistence theory[J]. Steel Research International, 2023,94(3):2200662. doi: 10.1002/srin.202200662 [17] Yang Xuemin, Li Jinyan, Zhang Meng, et al. Prediction model of sulfide capacity for CaO-FeO-Fe2O3-Al2O3-P2O5 slags in a large variation range of oxygen potential based on the ion and molecule coexistence theory[J]. Metallurgical and Materials Transactions B, 2014,45(6):2118-2137. [18] Yang Xuemin, Shi Chengbin, Zhang Meng, et al. A thermodynamic model for prediction of iron oxide activity in some FeO-containing slag systems[J]. Steel Research International, 2012,83(3):244-258. doi: 10.1002/srin.201100233 [19] Yang Xuemin, Li Jinyan, Chai Guoming, et al. A thermodynamic model for predicting phosphorus partition between CaO-based slags and hot metal during hot metal dephosphorization pretreatment process based on the ion and molecule coexistence theory[J]. Metallurgical and Materials Transactions B, 2016,47(4):2279-2301. [20] Li Jinyan, Zhang Mei, Guo Min, et al. Enrichment mechanism of phosphate in CaO-SiO2-FeO-Fe2O3-P2O5 steelmaking slags[J]. Metallurgical and Materials Transactions B, 2014,45(5):1666-1682. doi: 10.1007/s11663-014-0085-0 [21] Yang X M, Li J Y, Zhang M, et al. Prediction model of sulphur distribution ratio between CaO–FeO–Fe2O3–Al2O3–P2O5 slags and liquid iron over large variation range of oxygen potential during secondary refining process of molten steel based on ion and molecule coexistence theory[J]. Ironmaking & Steelmaking, 2016,43(1):39-55. [22] Lei Jialiu, Zhao Dongnan, Zhu Hangyu, et al. Thermodynamic model of desulfurization for CaO-SiO2-MgO-Al2O3-Na2O slag system based on IMCT theory[J]. Iron and Steel, 2019,54(3):35-41. (雷家柳, 赵栋楠, 朱航宇, 等. 基于IMCT理论的CaO-SiO2-MgO-Al2O3-Na2O渣系的脱硫热力学模型[J]. 钢铁, 2019,54(3):35-41.Lei Jialiu, Zhao Dongnan, Zhu Hangyu, et al. Thermodynamic model of desulfurization for CaO-SiO2-MgO-Al2O3-Na2O slag system based on IMCT theory[J]. Iron and Steel, 2019, 54(3): 35-41. [23] Zhang Meng, Guo Hanjie, Ding Rucai, et al. Thermodynamic model of sulfide capacity for CaO-SiO2-MgO-Al2O3 ironmaking slag system[J]. Journal of University of Science and Technology Beijing, 2011,33(9):1079-1084. (张盟, 郭汉杰, 丁汝才, 等. CaO-SiO2-MgO-Al2O3炼铁渣系硫化物容量的热力学模型[J]. 北京科技大学学报, 2011,33(9):1079-1084.Zhang Meng, Guo Hanjie, Ding Rucai, et al. Thermodynamic model of sulfide capacity for CaO-SiO2-MgO-Al2O3 ironmaking slag system[J]. Journal of University of Science and Technology Beijing, 2011, 33(9): 1079-1084. [24] Zhou Qiqi, Li Jipeng, Cheng Shusen, et al. Application of the IMCT model and KTH model in desulfurization practice of S50C steel[J]. Journal of Iron and Steel Research, 2020,32(4):297-303. (周旗旗, 李积鹏, 程树森, 等. IMCT模型和KTH模型在S50C钢脱硫实践中的应用[J]. 钢铁研究学报, 2020,32(4):297-303.Zhou Qiqi, Li Jipeng, Cheng Shusen, et al. Application of the IMCT model and KTH model in desulfurization practice of S50C steel[J]. Journal of Iron and Steel Research, 2020, 32(4): 297-303. [25] Sun Han, Yang Jian, Zhang Runhao, et al. Influence of temperature on dephosphorization at lower basicity and lower temperature based on industrial experiments and IMCT[J]. ISIJ International, 2022,62(6):1078-1090. [26] Wang Yunpeng, Cheng Guoguang, Li Shijian, et al. A novel model to design the equilibrium slag compositions for bearing steel: Verification and application[J]. ISIJ International, 2021,61(5):1514-1523. doi: 10.2355/isijinternational.ISIJINT-2020-671 [27] Li Bin, Li Lin, Guo Hanjie, et al. A phosphorus distribution prediction model for CaO–SiO2–MgO–FeO–Fe2O3–Al2O3–P2O5 slags based on the IMCT[J]. Ironmaking & Steelmaking, 2019,47(7):771-800. [28] Liu Liu. Smelting process of ultra-low phosphorus steel[J]. Special Steel, 2000(6):20-24. (刘浏. 超低磷钢的冶炼工艺[J]. 特殊钢, 2000(6):20-24.Liu Liu. Smelting process of ultra-low phosphorus steel[J]. Special Steel, 2000(6): 20-24. [29] Li Pengcheng, Yang Xuemin, Zhang Jian. Thermodynamic model of phosphorus distribution ratio in CaO-MgO-FeO-Fe2O3-SiO2 steelmaking slag system[J]. Journal of University of Science and Technology Beijing, 2014,36(12):1608-1614. (李鹏程, 杨学民, 张鉴. CaO-MgO-FeO-Fe2O3-SiO2炼钢渣系磷分配比的热力学模型[J]. 北京科技大学学报, 2014,36(12):1608-1614.Li Pengcheng, Yang Xuemin, Zhang Jian. Thermodynamic model of phosphorus distribution ratio in CaO-MgO-FeO-Fe2O3-SiO2 steelmaking slag system[J]. Journal of University of Science and Technology Beijing, 2014, 36(12): 1608-1614. [30] Li Lin. Fundamental research on the HIsmelt ironmaking process[D]. Beijing: University of Science and Technology Beijing, 2020. (李林. HIsmelt炼铁工艺的基础研究[D]. 北京: 北京科技大学, 2020.Li Lin. Fundamental research on the HIsmelt ironmaking process[D]. Beijing: University of Science and Technology Beijing, 2020. [31] Zhou Chaogang, Li Jing, Shi Chengbin, et al. Dependence of temperature and slag composition on dephosphorization at the first deslagging in BOF steelmaking process[J]. High Temperature Materials and Processes, 2016,35(4):433-440. doi: 10.1515/htmp-2014-0187 -



 下载:
下载: