Preparation of VN via core-shell precursor method under the intervention of dispersants
-
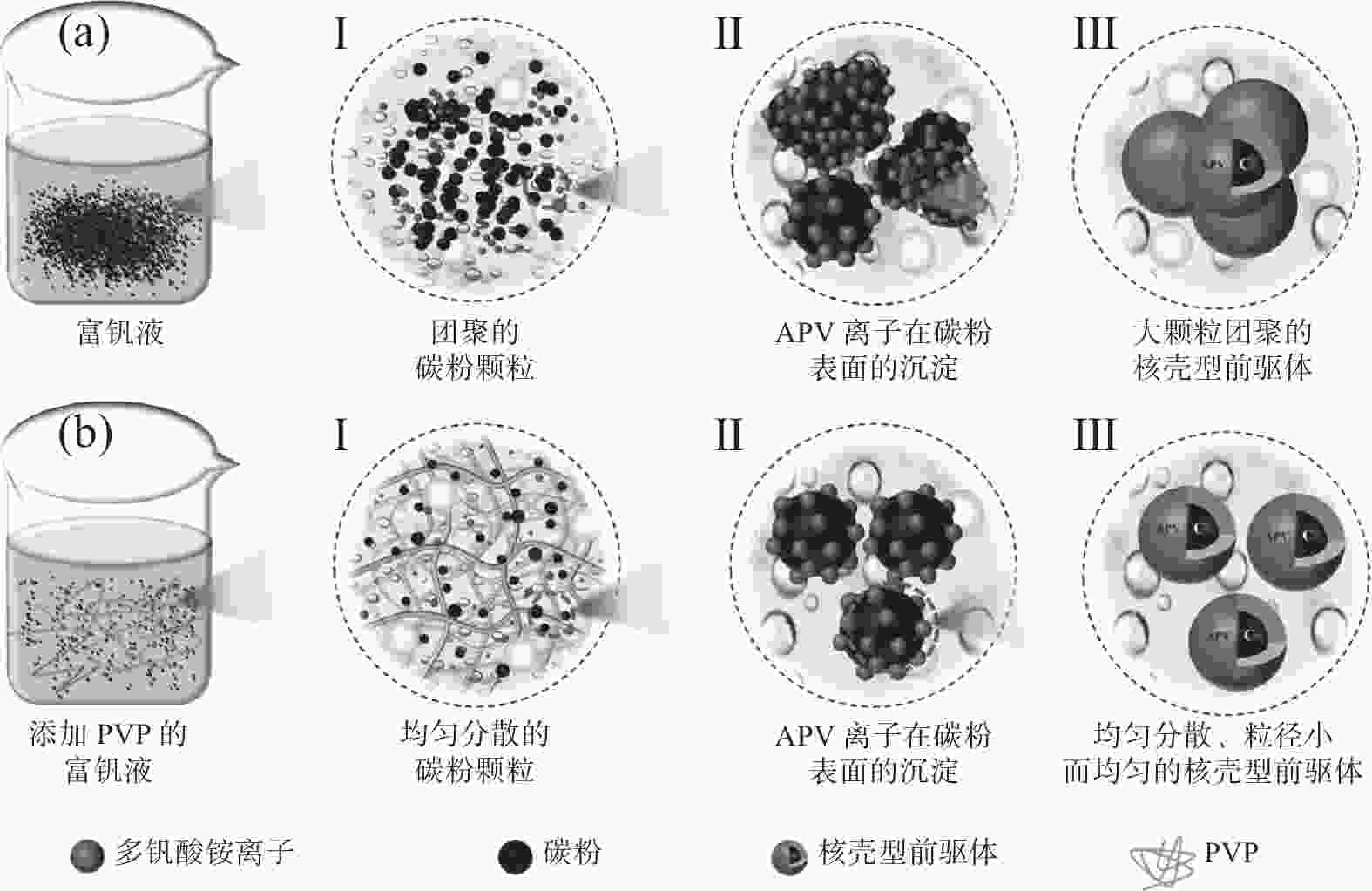
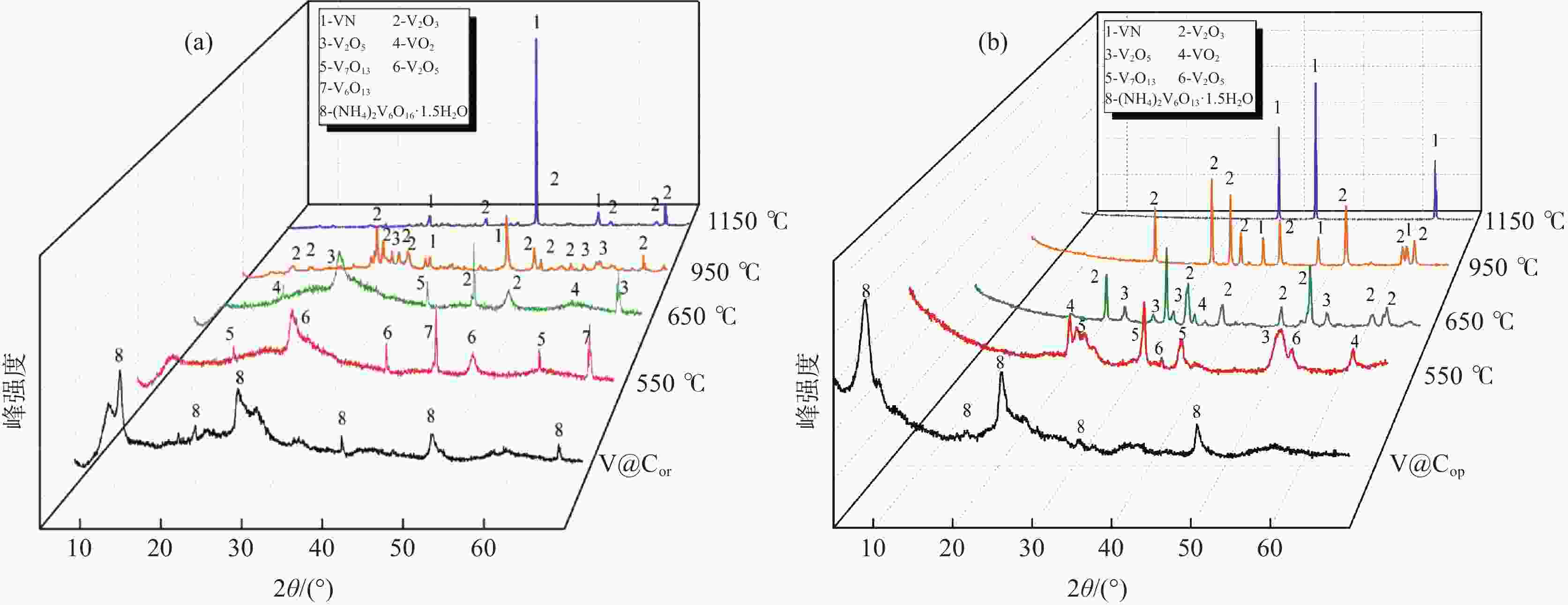
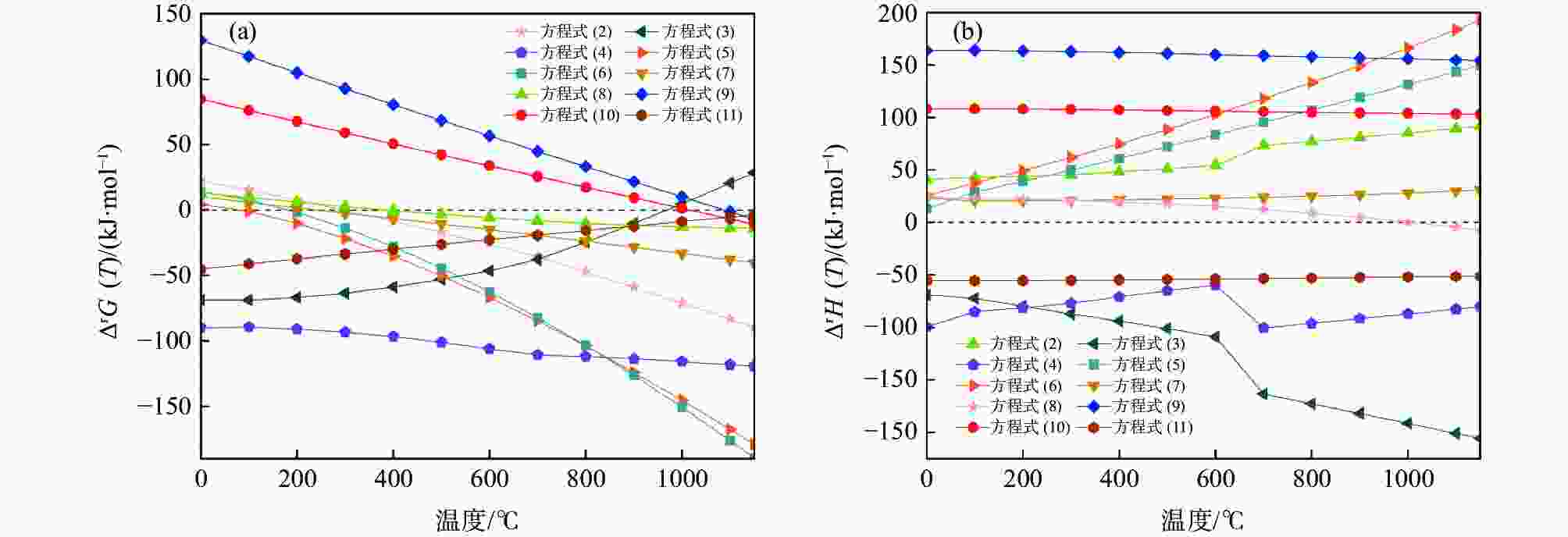
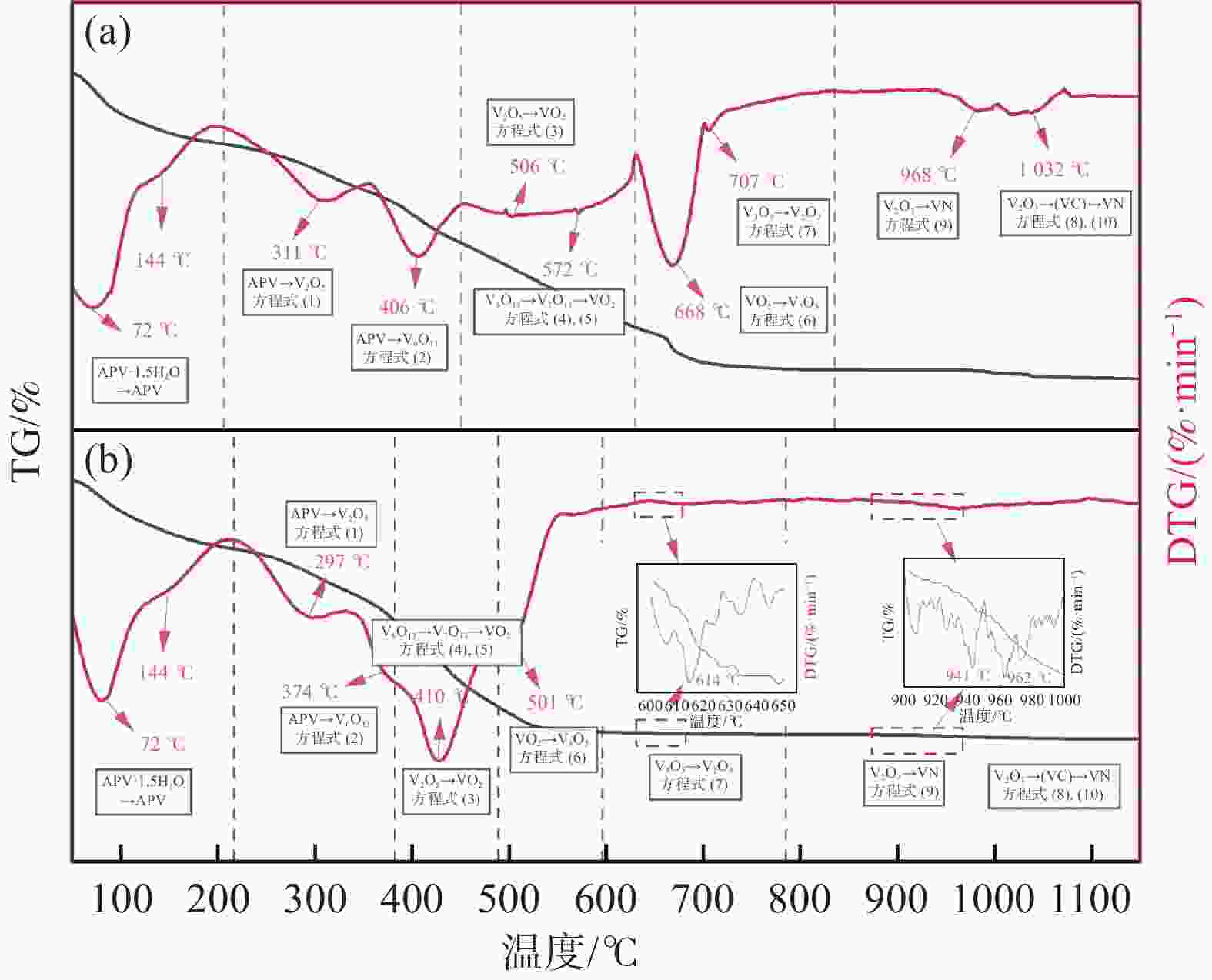
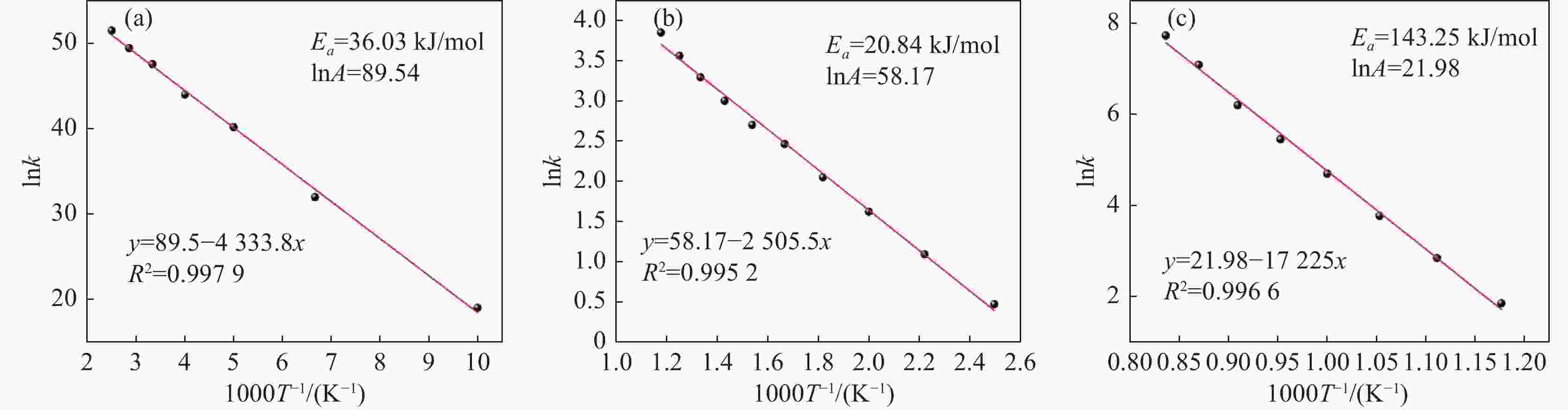
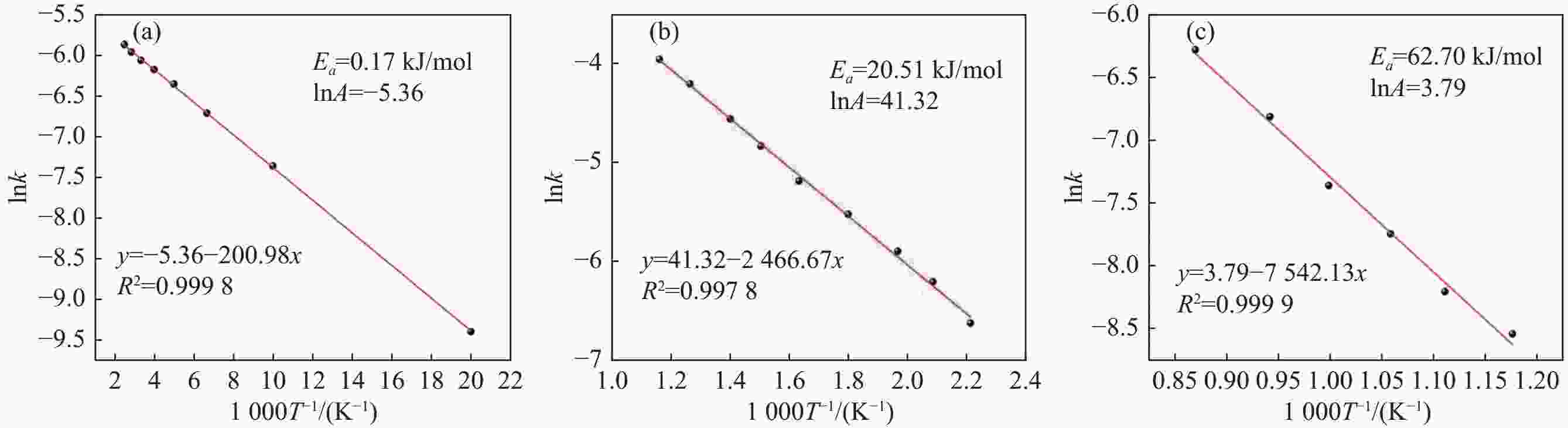
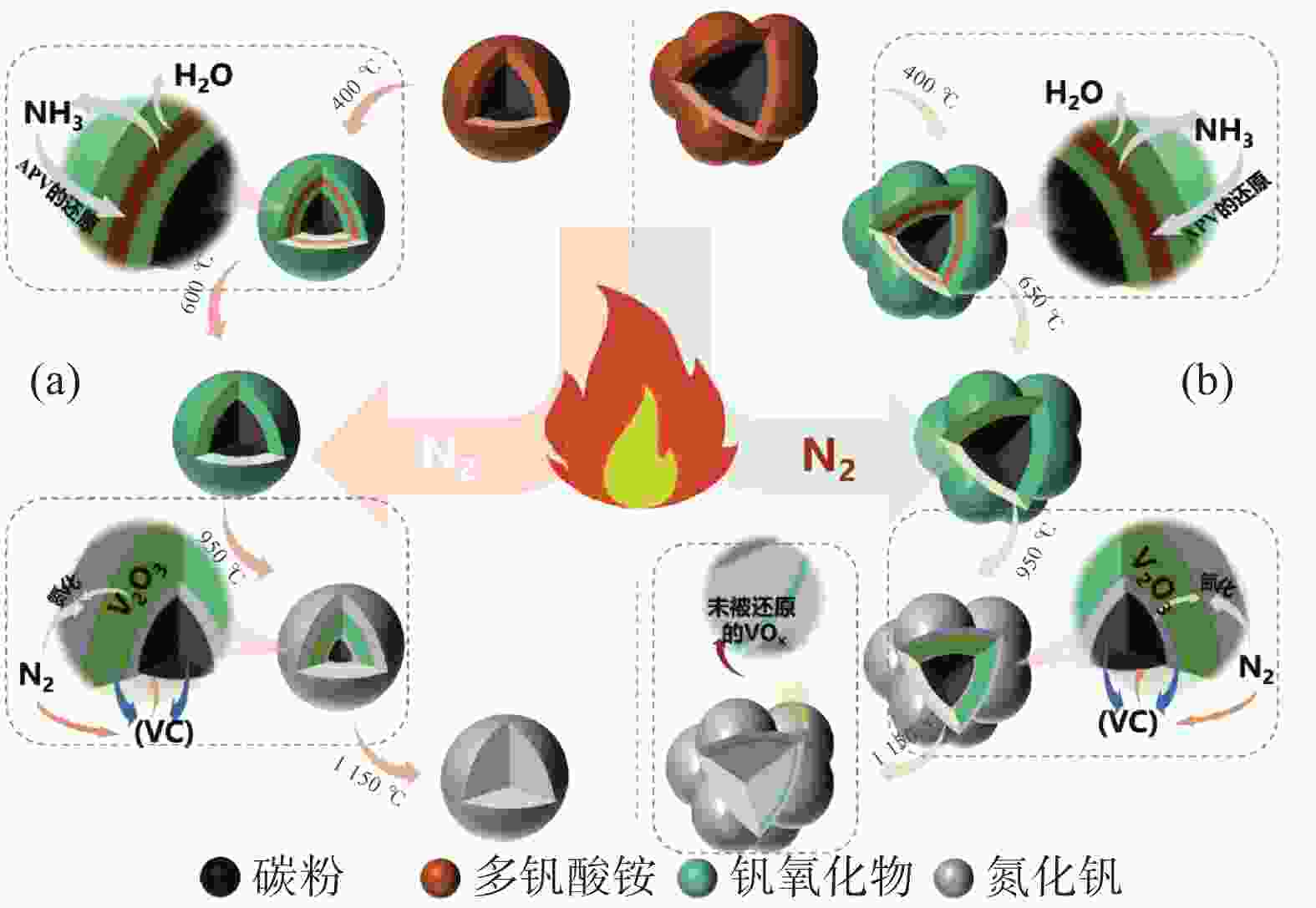
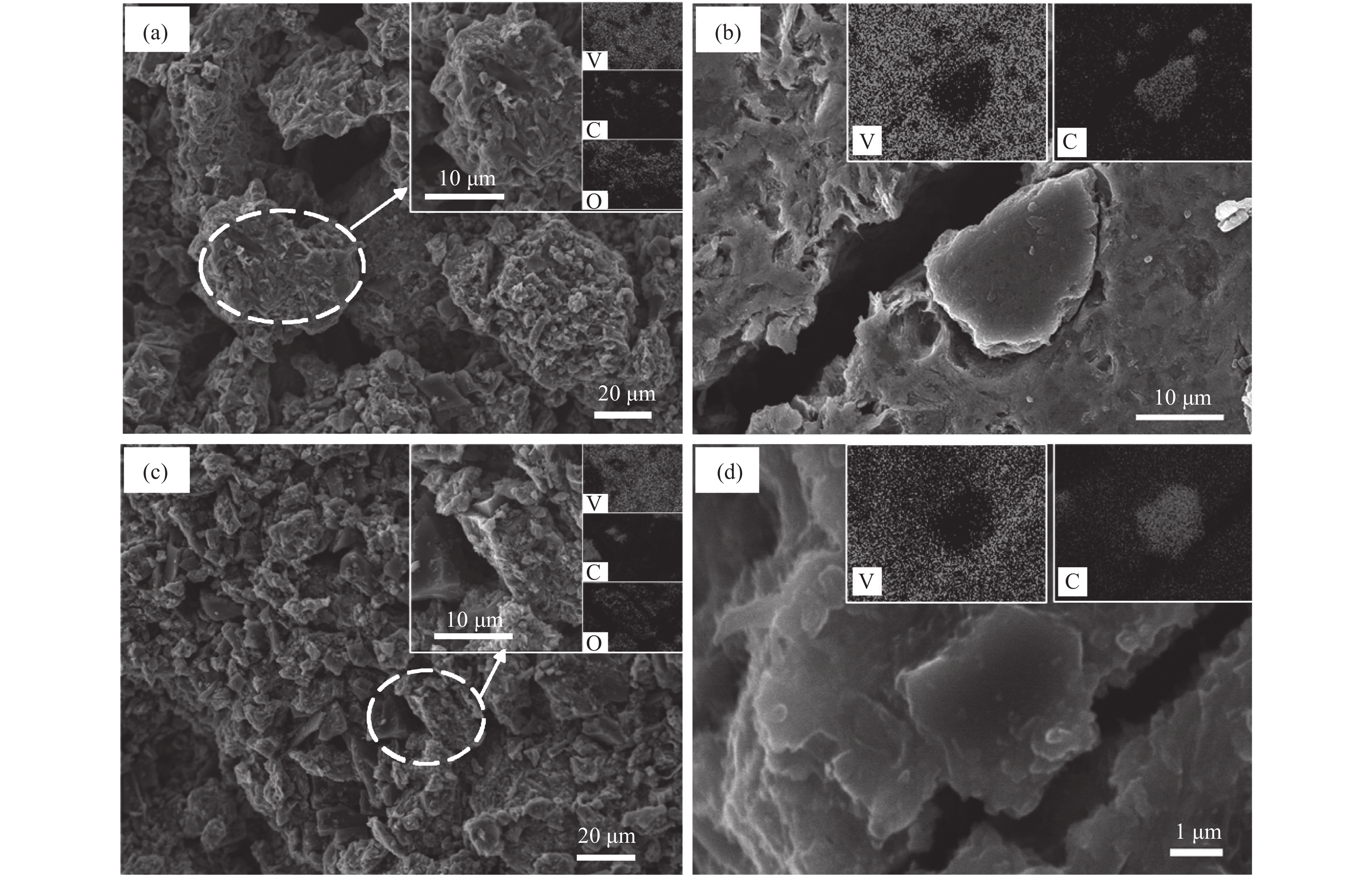
摘要: 采用聚乙烯吡咯烷酮(PVP)优化核壳型钒碳包裹前驱体结构,热处理前驱体获得满足国标VN16牌号的氮化钒(VN)。PVP的引入促进了碳粉在富钒溶液中的均匀分散,有利于多聚钒酸铵(APV) 离子的氢键化,使其吸附于碳粉表面成核和生长,制备的前驱体有包覆完整稳定且厚度均匀适中的APV外壳、碳粉内核及小且均匀的粒径分布。在还原氮化过程中,前驱体到VN的相变过程为:APV→V2O5→V6O13→V7O13→VO2→V3O5→V2O3→(VC) →VN。优化后的前驱体因其细密的核壳包覆结构和均匀的粒度分布,形成了更稳定的相反应界面和更多的反应活性位点,降低了各阶段的反应活化能(Ea),使还原氮化效率更高,更易向低价VOx和VN转变。与现行碳热还原工艺相比,反应时间缩短75%,N2流量由300 mL/min降低至200 mL/min,耗量约降低40%,显著降低生产成本。Abstract: In this study, polyvinyl pyrrolidone (PVP) is used to optimize the core-shell V@C precursor structure, and the precursor is heat-treated to obtain vanadium nitride (VN) up to the National Standard VN16 grade of China. The addition of PVP promotes both the uniform dispersion of the carbon powders in the vanadium rich solution and facilitates the hydrogen bonding of ammonium polyvanadate (APV) ions, which are adsorbed on the surface of carbon powders for nucleation and growth. The as-prepared precursor by adding PVP has better encapsulated and stable carbon powder core and APV shell with uniform and moderate thickness, as well as small and homogeneous particle size distribution. In the nitridation and reduction process, the phase transition from precursor to VN is as follows: APV → V2O5 → V6O13 → V7O13 → VO2 → V3O5 → V2O3 → (VC) → VN. Due to its more stable core-shell coating structure and more uniform particle size distribution, the optimized precursor forms a more stable phase reaction interface and more active reaction sites, which reduces the reaction activation energy (Ea) at each stage, and makes it more efficient in reducing and nitriding and easier to transition to low-valent VOx and VN. In comparison with current carbothermal reduction process, the reaction time is shortened by 75%, and the flow rate of N2 is reduced from 300 mL/min to 200 mL/min, the usage of N2 is reduced by 40%, significantly reducing production costs.
-
Key words:
- vanadium nitride /
- PVP /
- core-shell precursor /
- phase reaction interface
-
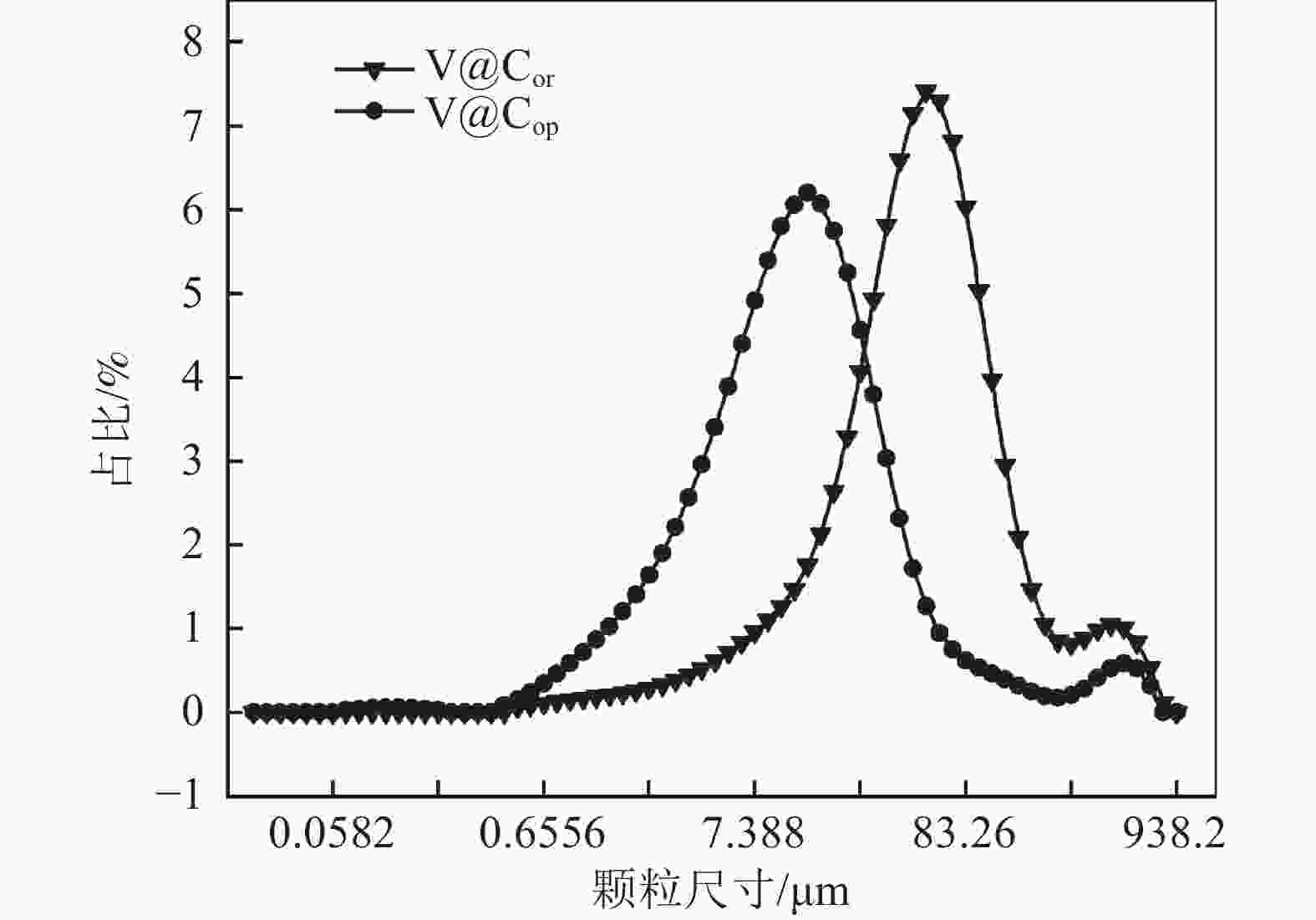
表 1 前驱体的激光粒度分布参数
Table 1. Laser particle size distribution parameters of different precursors
前驱体 比表面积
/(m2·kg−1)D(3, 2)
/μmD(4, 3)
/μmDv (50)
/μmDv (90)
/μmV@Cor 326.523 18.38 76.33 46.54 147.03 V@Cop 1272.47 4.92 29.07 10.92 40.53 表 2 VN的化学成分
Table 2. Chemical composition of vanadium nitride
% VN产品 V N C S P V@Cor 79.68 16.74 3.46 0.08 0.04 V@Cop 78.93 17.91 2.3 0.07 0.02 国标VN16 77.0~81.0 14.0~<18.0 ≤6.0 ≤0.1 ≤0.06 -
[1] Li Hongyi , Wang Chengjie, Lin Minmin, et al. Green one-step roasting method for efficient extraction of vanadium and chromium from vanadium-chromium slag[J]. Powder Technology, 2020,360:503-508. doi: 10.1016/j.powtec.2019.10.074 [2] Li Hongyi, Yang Yang, Zhang Meng, et al. A novel anion exchange method based on in situ selectively reductive desorption of Cr(VI) for its separation from V(V): Toward the comprehensive use of hazardous wastewater[J]. J. Hazard. Mater. , 2019, 368: 670-679. [3] Luo Daibing, Ma Wangjing, Wu Liangzhuan, et al. Flexible PET substrate coated with V2O5 film with porous network prepared by EPLSD method[J]. Appl. Surf. Sci. , 2021, 538 : 148053. [4] Amina Shafique, Muhammad Ashar Naveed, Sumbel Ijaz, et al. Highly efficient vanadium nitride based metasurface absorber/emitter for solar-thermophotovoltaic system[J]. Mater. Today Common. , 2023, 34: 105416. [5] Shang Guangmin, Liu Hongyu, Deng Weijie, et al. The addition of anadium and nitride alloy on the solidification structure and hardness of M2 high-speed steel[J]. Iron Steel Vanadium Titanium, 2024,45(2):182-189. (商光敏, 刘宏玉, 邓玮杰,等. 添加钒氮合金对M2高速钢凝固组织与硬度的影响[J]. 钢铁钒钛, 2024,45(2):182-189.Shang Guangmin, Liu Hongyu, Deng Weijie, et al. The addition of anadium and nitride alloy on the solidification structure and hardness of M2 high-speed steel[J]. Iron Steel Vanadium Titanium, 2024, 45(2): 182-189. [6] Li Runkang, Lu Jiaqi, Li Chaojie, et al. Mesoporous vanadium nitride nanofiber@N-doped carbon with excellent microwave absorption and anti-corrosion[J]. Colloids Surf. A, 2024,686:133420. doi: 10.1016/j.colsurfa.2024.133420 [7] Zhang Dongbin, Chang Zhi, Teng Aijun, et al. Regulation on electronic structure of VN-based materials for enhanced supercapacitor performances[J]. Iron Steel Vanadium Titanium, 2022,43(5):45-51. (张东彬, 常智, 滕艾均, 等. VN基材料的电子结构调控和超电容性能研究[J]. 钢铁钒钛, 2022,43(5):45-51.Zhang Dongbin, Chang Zhi, Teng Aijun, et al. Regulation on electronic structure of VN-based materials for enhanced supercapacitor performances[J]. Iron Steel Vanadium Titanium, 2022, 43(5): 45-51. [8] Holz L I V, Loureiro F J A, Graca V C D, et al. Vanadium (oxy)nitride as a new category of anode for direct ammonia solid oxide fuel cells cells[J]. Renewable Energy, 2022, 201: 124-130. [9] Wu Ziqiang, Chen Qian, Li Changdian, et al. Hydrogel-derived nitrogen-doped porous carbon framework with vanadium nitride decoration for supercapacitors with superior cycling performance[J]. J. Mater. Sci. Technol., 2023,155:167-174. doi: 10.1016/j.jmst.2023.01.031 [10] Ye Miaoting, Bu Naijing, Chen Lai, et al. Study on reaction mechanism for the synthesis of vanadium nitride by carbothermic reduction nitridation method[J]. Ceram. Int., 2024,50(5):7458-7468. doi: 10.1016/j.ceramint.2023.12.040 [11] Chen Zhichao, Xue Zhengliang, Wang Wei, et al. One-step method of carbon thermal reduction and nitride to produce vanadium nitrogen alloy[J]. Adv. Mat. Res. , 2012, 476-478: 194-198. [12] Han Jinglei, Zhang Yimin, Liu Tao, et al, Preparation of vanadium nitride using a thermally processed precursor with coating structure[J]. Metals, 2017,7:360. doi: 10.3390/met7090360 [13] Wen Ailian, Cai Zhenlei, Zhang Yimin, et al. A novel method of preparing vanadium-based precursors and their enhancement mechanism in vanadium nitride preparation[J]. RSC Adv., 2022,12:13093-13102. [14] Sun Chunyu, Su Haitang, Han Xuelian, et al. Influence of polymer dispersants on dispersion effect of polymethyl methacrylate microspheres by dispersion polymerization[J]. Polymer Materials Science & Engineering, 2023,39(7):1-7. (孙春雨, 苏海棠, 韩雪莲, 等. 高分子分散剂对分散聚合制备聚甲基丙烯酸甲酯微球分散效果的影响[J]. 高分子材料科学与工程, 2023,39(7):1-7.Sun Chunyu, Su Haitang, Han Xuelian, et al. Influence of polymer dispersants on dispersion effect of polymethyl methacrylate microspheres by dispersion polymerization[J]. Polymer Materials Science & Engineering, 2023, 39(7): 1-7. [15] Shang Xu, Jing Xiwei, Xu Jian, et al. Influence of polyvinylpyrrolidone with different molecular weights on the dispersion of multiwalled carbon nanotubes[J]. Journal of East China University of Science and Technology, 2019,45(6):883-890. (尚旭, 景希玮, 徐健,等. 不同分子量聚乙烯吡咯烷酮对多壁碳纳米管分散性能的影响[J]. 华东理工大学学报(自然科学版), 2019,45(6):883-890.Shang Xu, Jing Xiwei, Xu Jian, et al. Influence of polyvinylpyrrolidone with different molecular weights on the dispersion of multiwalled carbon nanotubes[J]. Journal of East China University of Science and Technology, 2019, 45(6): 883-890. [16] Franco-Ulloa S, Tatulli G, Bore S L, et al. Dispersion state phase diagram of citrate-coated metallic nanoparticles in saline solutions[J]. Nat Commun, 2020, 11 : 5422. [17] Rezvani R, Nabizadeh A, Amin Tutunchian M. The effect of particle size distribution on shearing response and particle breakage of two different calcareous soils[J]. Eur. Phys. J. Plus, 2021, 136: 1008. [18] Koczkur K M, Mourdikoudis S, Polavarapu L, et al. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis[J]. Dalton Trans. 2015, 44: 17883-17905. [19] Kang Liwu, Zeng Jun, Huang Yonggang. The oxidation dynamics of ilmenite[J]. Iron Steel Vanadium Titanium, 2017,38(6):23-26. (康立武, 曾俊, 黄永刚. 钛铁矿氧化动力学研究[J]. 钢铁钒钛, 2017,38(6):23-26.Kang Liwu, Zeng Jun, Huang Yonggang. The oxidation dynamics of ilmenite[J]. Iron Steel Vanadium Titanium, 2017, 38(6): 23-26. [20] Li Wenbo, Chen Jijia, Cheng Shaokai, et al. Thermal decomposition mechanism and kinetics of bastnaesite in suspension roasting process: A comparative study in N2 and air atmospheres[J]. J. of Rare Earth, 2023, 42(9):1809-1816. [21] Zhang Qi, Sun Yongsheng, Han Yuexin, et al. Pyrolysis behavior of a green and clean reductant for suspension magnetization roasting[J]. Journal of Cleaner Production, 2020,268:122173. doi: 10.1016/j.jclepro.2020.122173 [22] Zhang Qi, Sun Yongsheng, Qin Yonghong, et al. Siderite pyrolysis in suspension roasting: An in-situ study on kinetics, phase transformation, and product properties[J]. J. Ind. Eng. Chem., 2022,50:7458-7468. [23] Mao Zhongtian, Campbell Charles T. Apparent activation energies in complex reaction mechanisms: a simple relationship via degrees of rate control[J]. ACS Catal, 2019,9:9465-9473. doi: 10.1021/acscatal.9b02761 -





 下载:
下载: