Research on the vanadium extraction from deactivated sulfuric acid catalyst featured with two-step and selectively
-
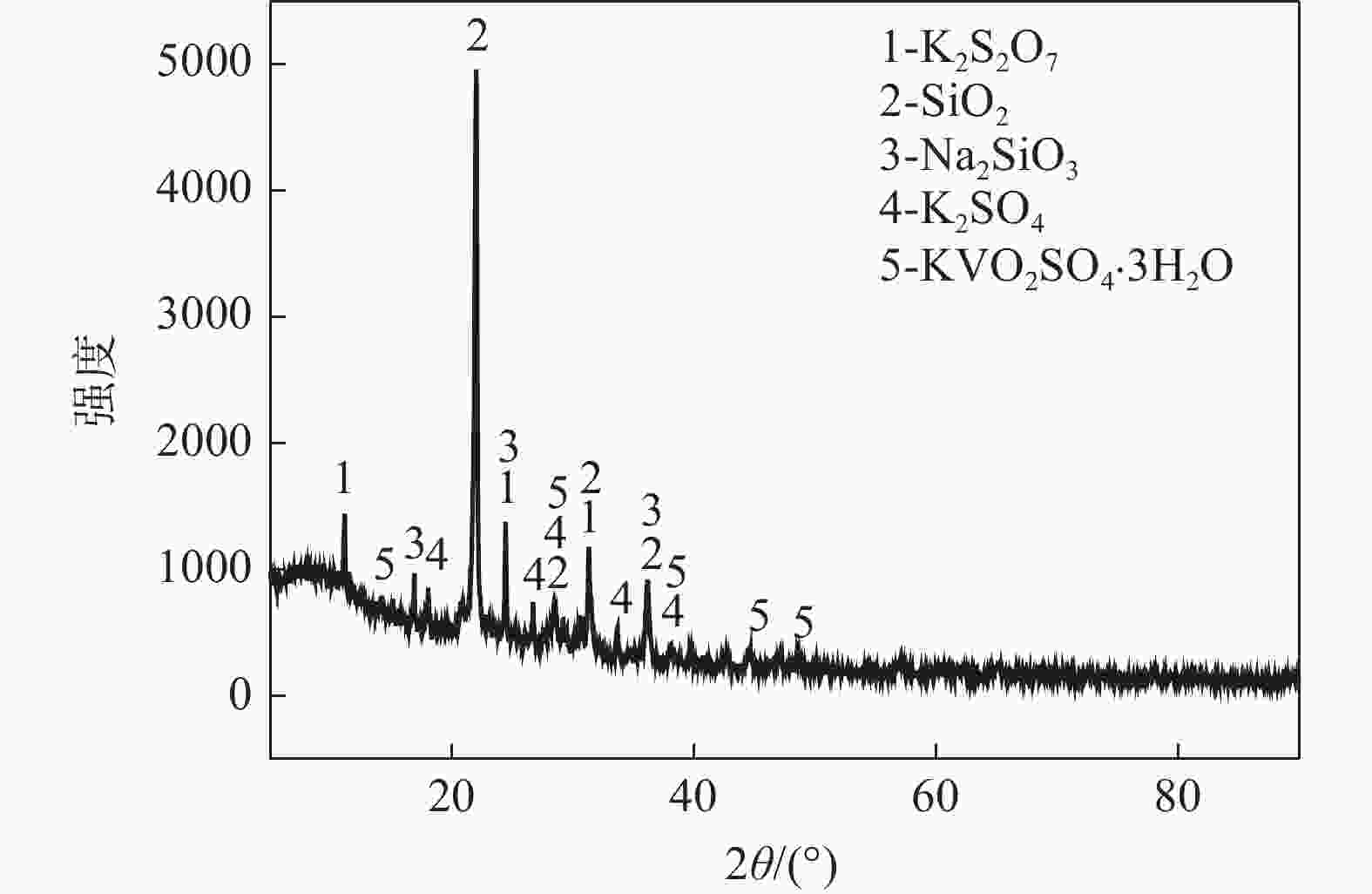
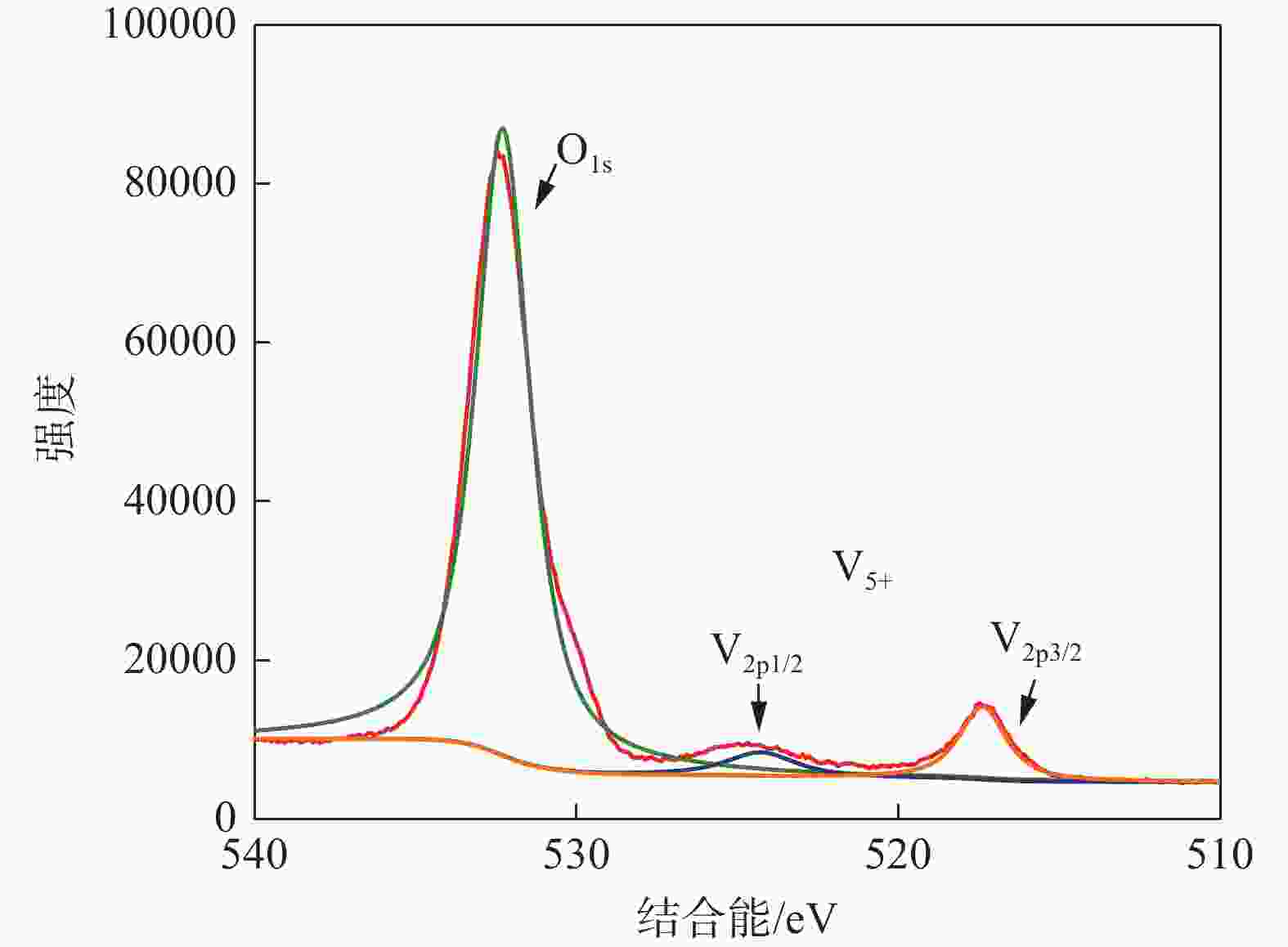
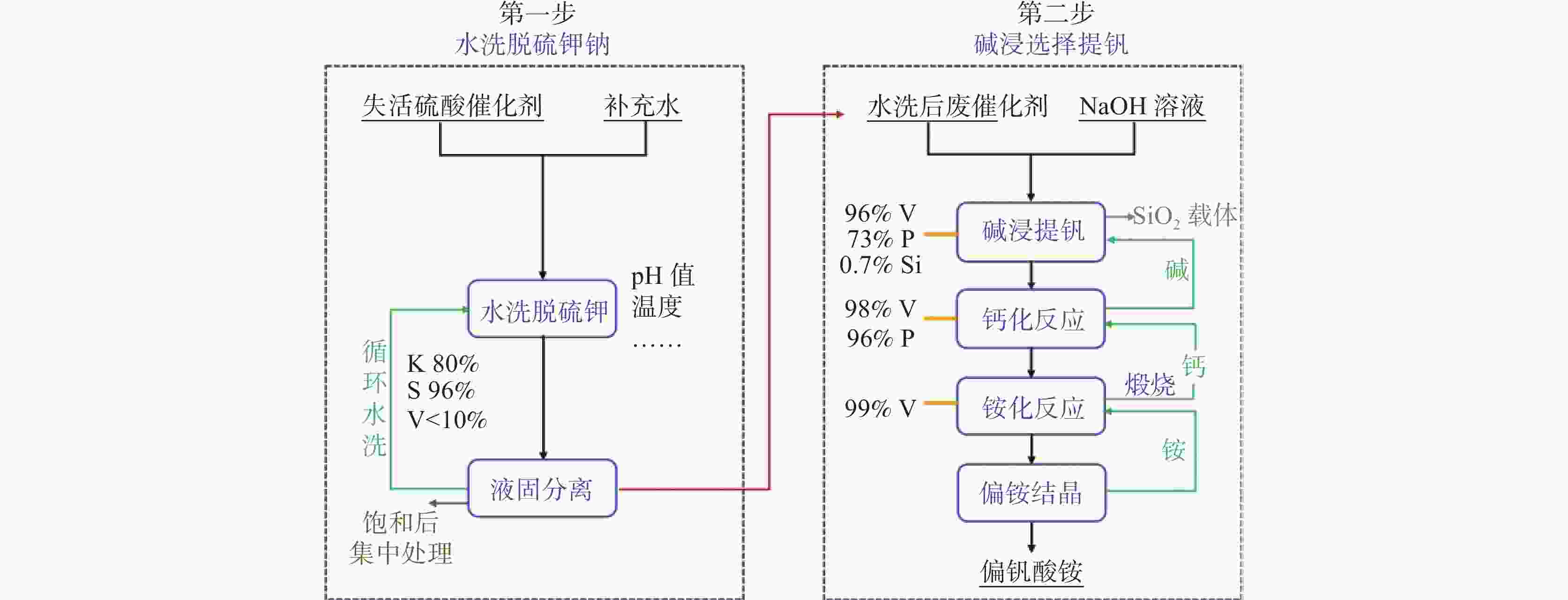
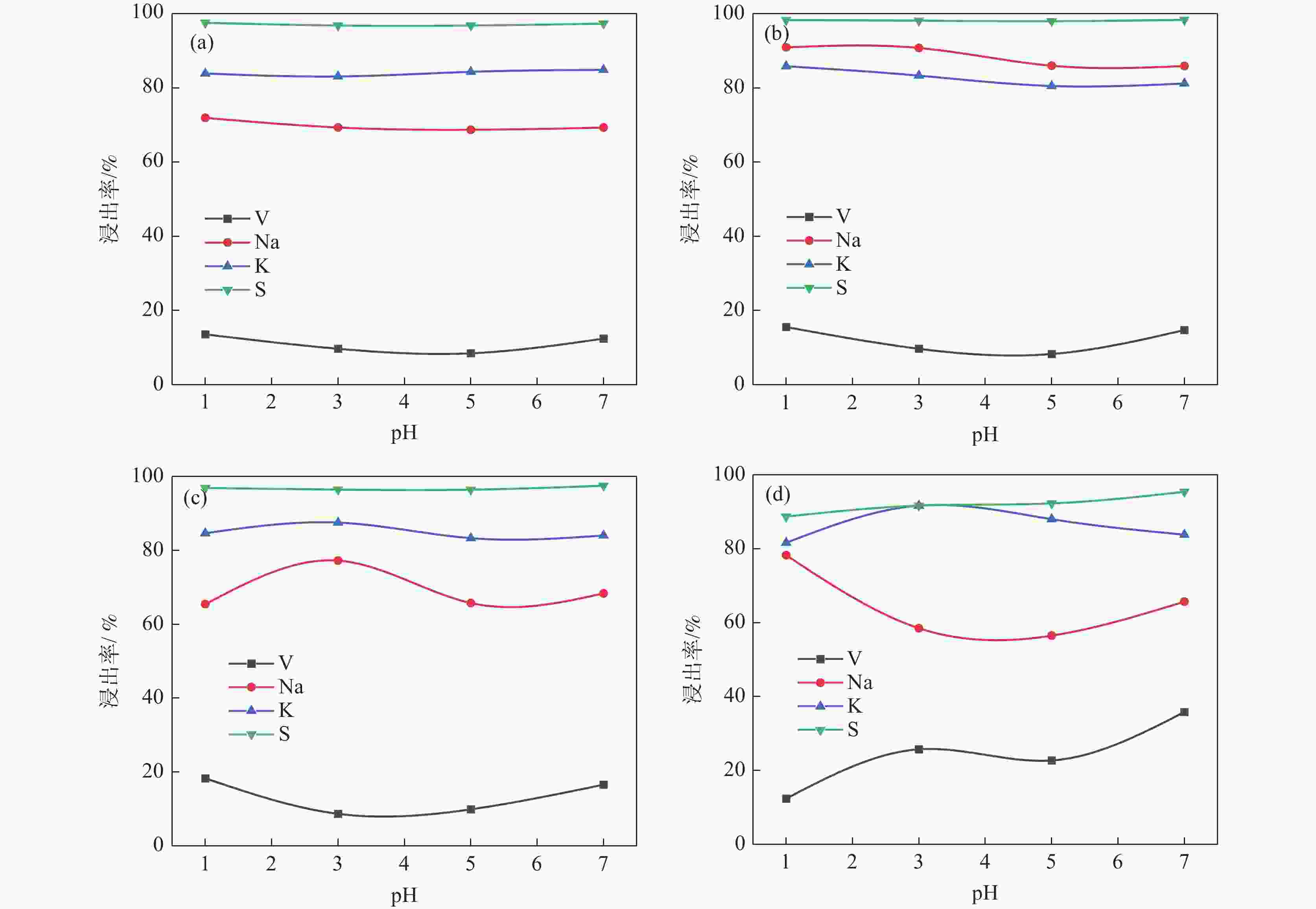
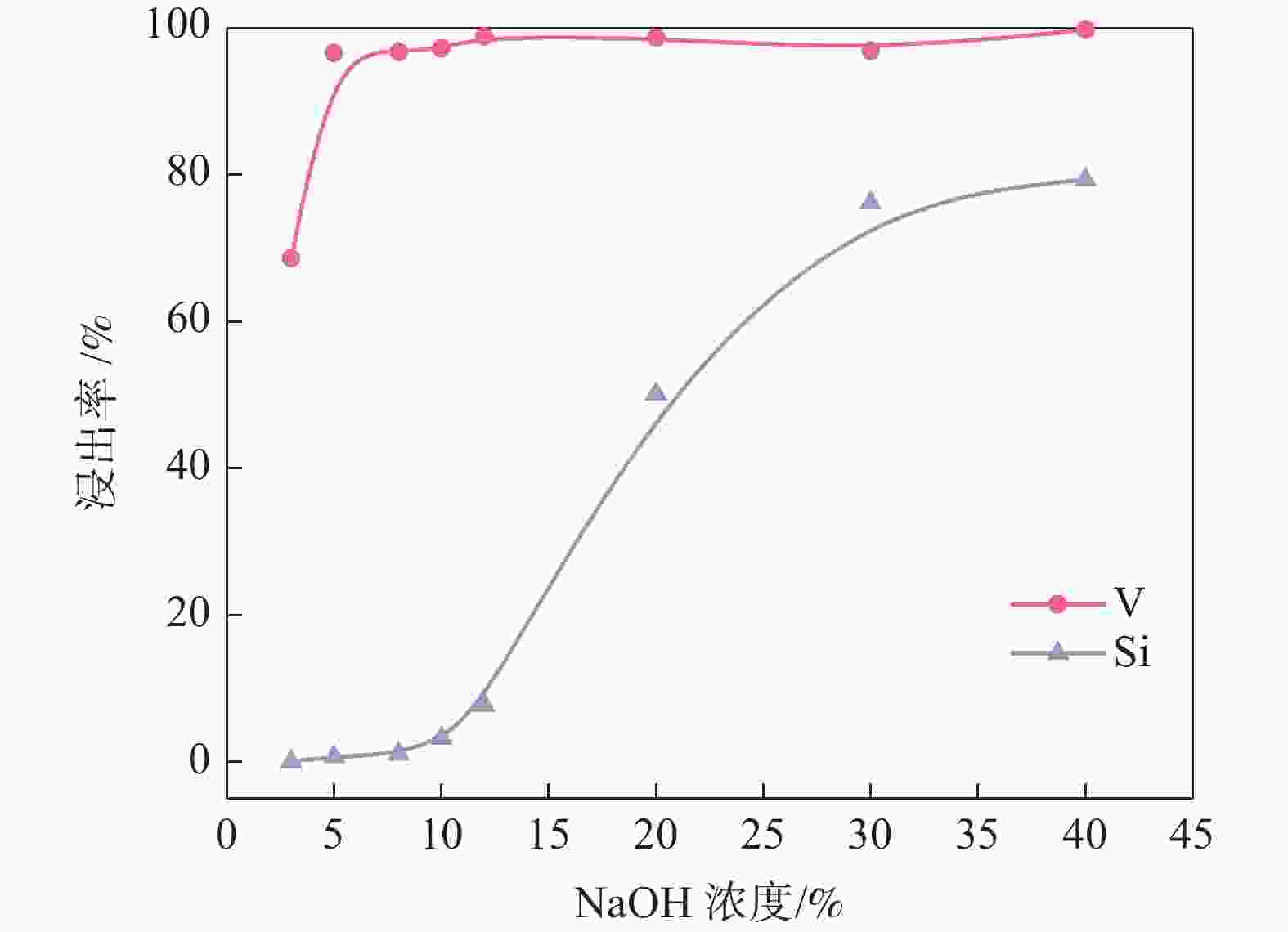
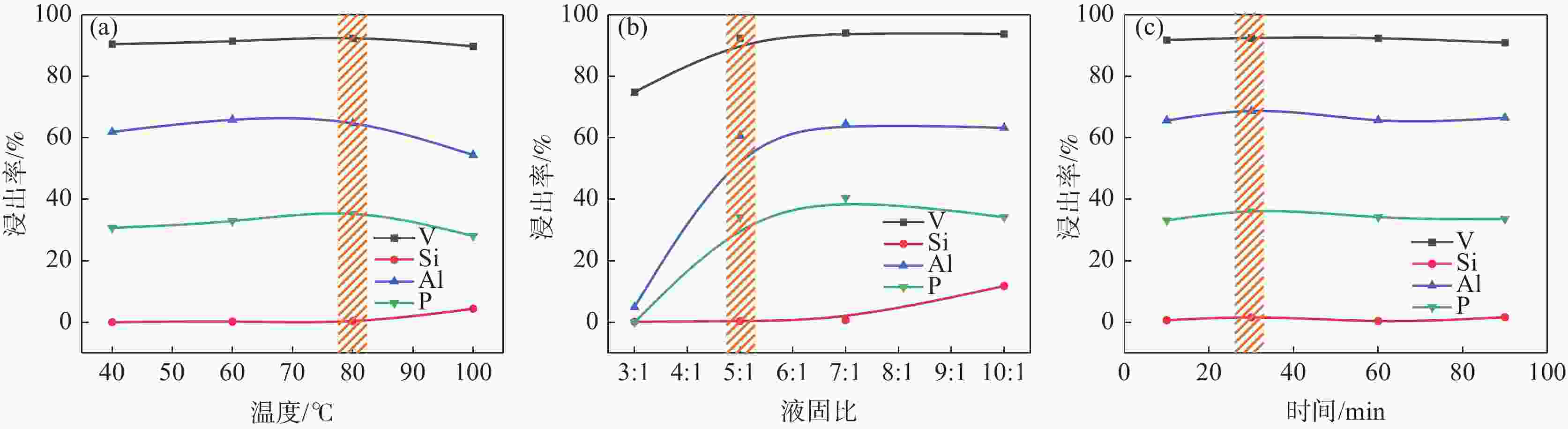
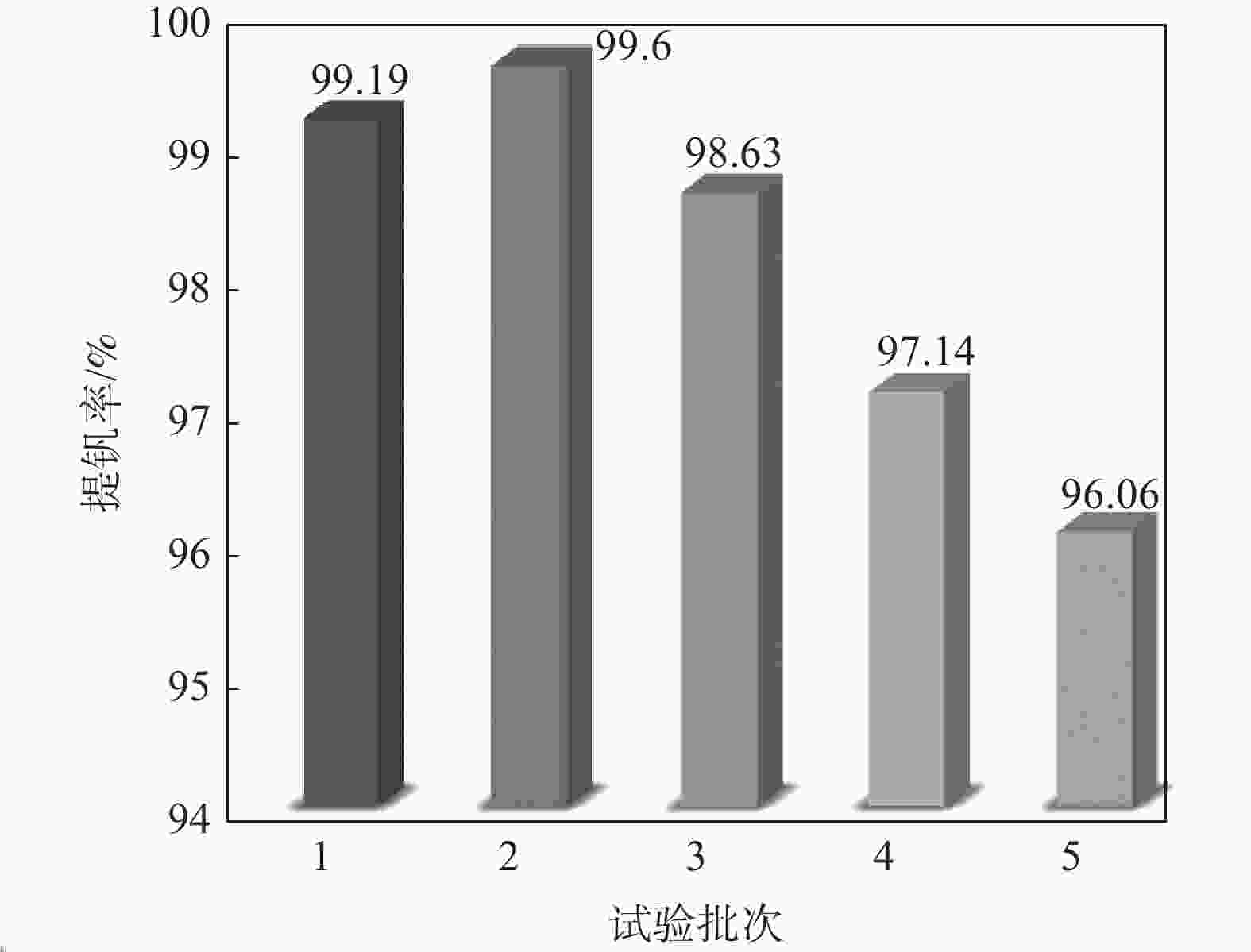
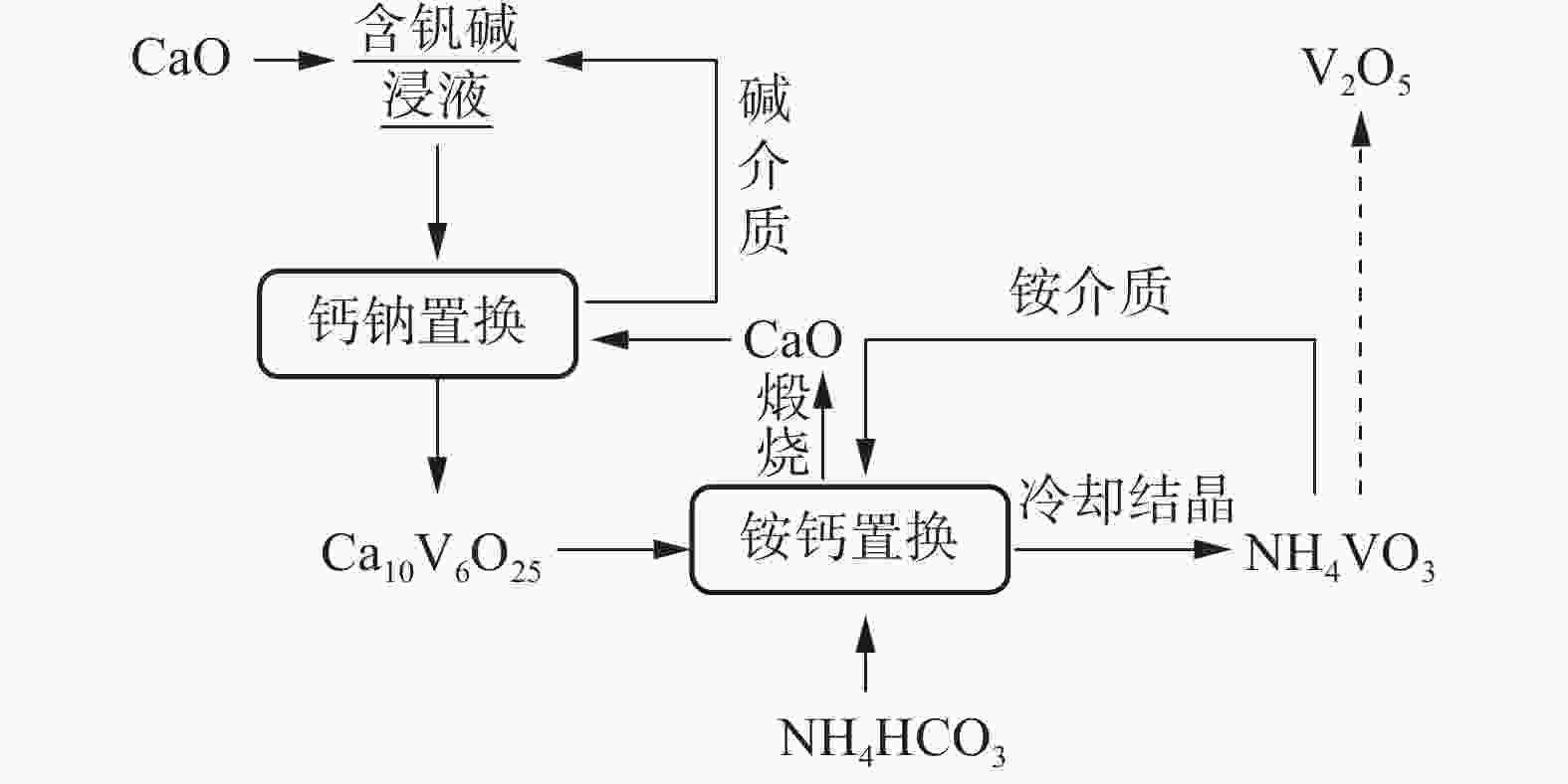
摘要: 针对失活硫酸催化剂直接酸浸、碱浸工艺选择性差,后续分离代价高、介质难回用的问题,使用两步法选择性提钒新工艺,利用钾、硫的强水溶性特点,研究了水洗温度、pH值对钾、硫浸出的影响,发现在温度40~60 ℃、pH值3~5水洗条件下,钾、硫的浸出率分别达到80%、96%以上,而钒的损失率控制在10%以内,实现了钒与钾、硫的选择性分离;第二步利用钒、硅在碱介质中的溶出特性,研究了碱浸温度、时间、液固比及碱液浓度对提钒效果的影响,发现采用5% NaOH溶液在80 ℃、L/S=5条件下反应30 min可实现失活硫酸催化剂中钒95%以上的浸出,而硅基本不浸出,实现了钒、硅的选择性分离。含钒浸出液可经钙化-铵化工艺制备得到偏钒酸铵产品,NaOH介质返回提钒阶段循环使用,介质封闭。Abstract: Based on the limited selectivity exhibited by current direct acid leaching and alkaline leaching processes for deactivated sulfuric acid catalysts, resulting in costly subsequent separation and challenging medium reuse, a new two-step process for selective extraction of vanadium was adopted. Using the strong water solubility of potassium and sulfur, the influence of washing temperature and pH value on leaching of potassium and sulfur was studied. It was found that potassium and sulfur can be removed more than 80% and 96% respectively by water washing under the condition of temperature 40~60℃ and pH 3~5, while the loss rate of vanadium was controlled within 10%. Thus, the selective separation of vanadium from potassium and sulfur was realized. In the second step, based on the leaching characteristics of vanadium and silicon in an alkali medium, the effects of leaching temperature, time, liquid-solid ratio and alkali concentration on vanadium extraction were studied. It was found that after reaction with 5% NaOH solution at 80 ℃ and L/S = 5 for 30 min, more than 95% vanadium was deactivated from the sulfuric acid catalyst, while the silicon leaching rate kept almost zero. The vanadium-containing leaching solution can be handled by calcification and ammonium process to obtain ammonium metavanadate products. The NaOH solution can be recycled to vanadium extraction step and wastewater zero emission was achieved.
-
表 1 失活硫酸催化剂成分分析结果
Table 1. Result of ICP analysis of deactivated sulfuric acid catalyst
% Na2O SiO2 V2O5 K2O MgO CaO TiO2 Fe2O3 Al2O3 P2O5 As2O5 SO3 5.31 53.04 5.23 11.73 0.17 0.20 0.10 1.21 1.62 1.45 0.02 19.92 表 2 最佳水洗条件下失活硫酸催化剂水洗液成分
Table 2. Composition of water lotion of deactivated sulfuric acid catalyst under optimal water washing condition
温度/℃ pH 含量/(g·L−1) Na2O SiO2 V2O5 K2O MgO CaO TiO2 Fe2O3 Al2O3 P2O5 S 40 3 11.56 <检测下限 0.70 22.00 0.50 0.42 <检测下限 0.31 1.92 0.07 17.03 5 13.60 <检测下限 0.17 23.41 0.49 0.42 <检测下限 0.02 1.85 0.03 17.11 60 3 8.50 <检测下限 0.10 19.80 0.38 0.30 <检测下限 0.32 1.39 <检测下限 13.85 5 8.54 <检测下限 0.24 19.25 0.36 0.30 <检测下限 <检测下限 1.05 0.02 13.55 表 3 NaOH浸液钙化铵化成分变化
Table 3. Changes of calcified ammonium compositions in NaOH infusion
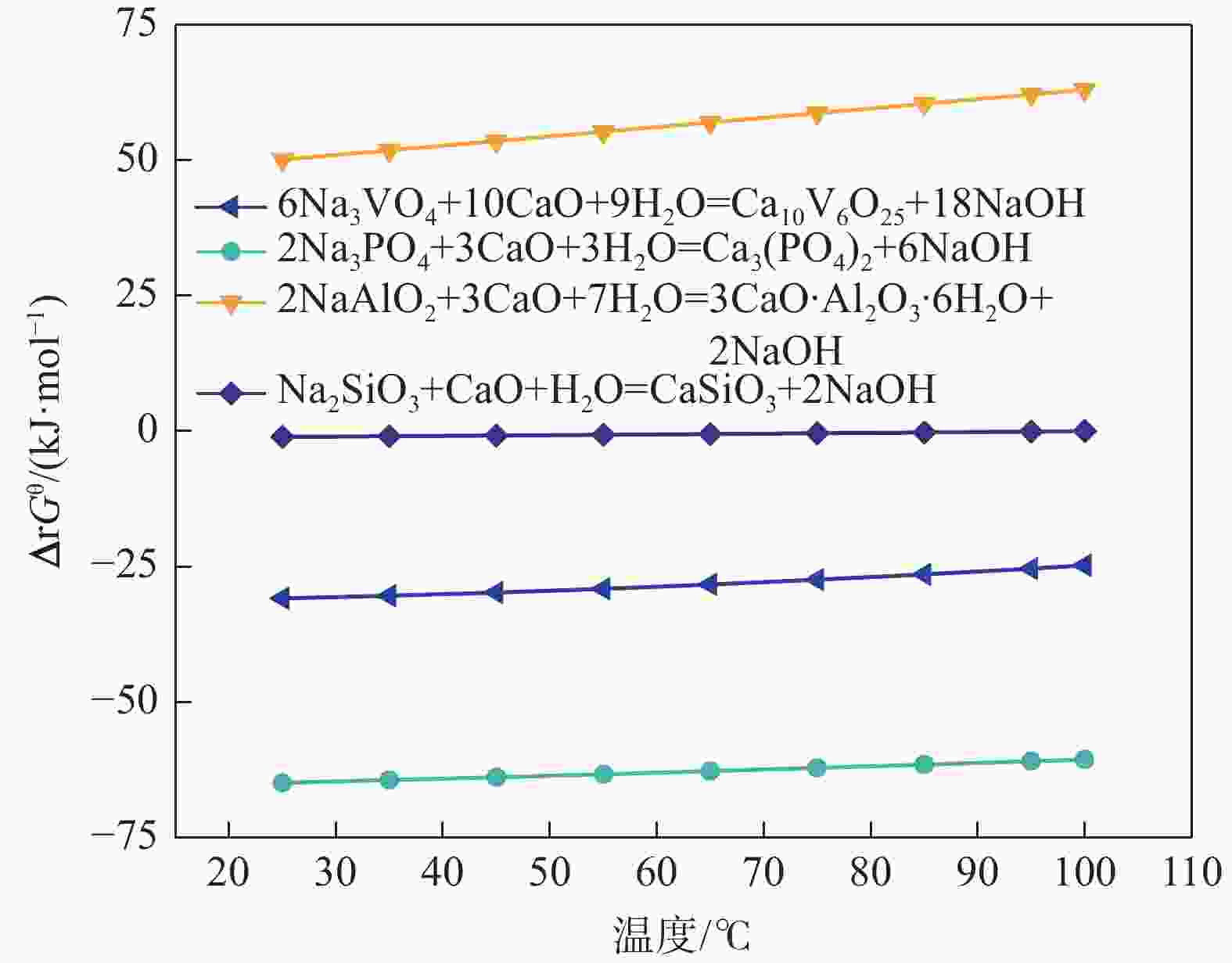
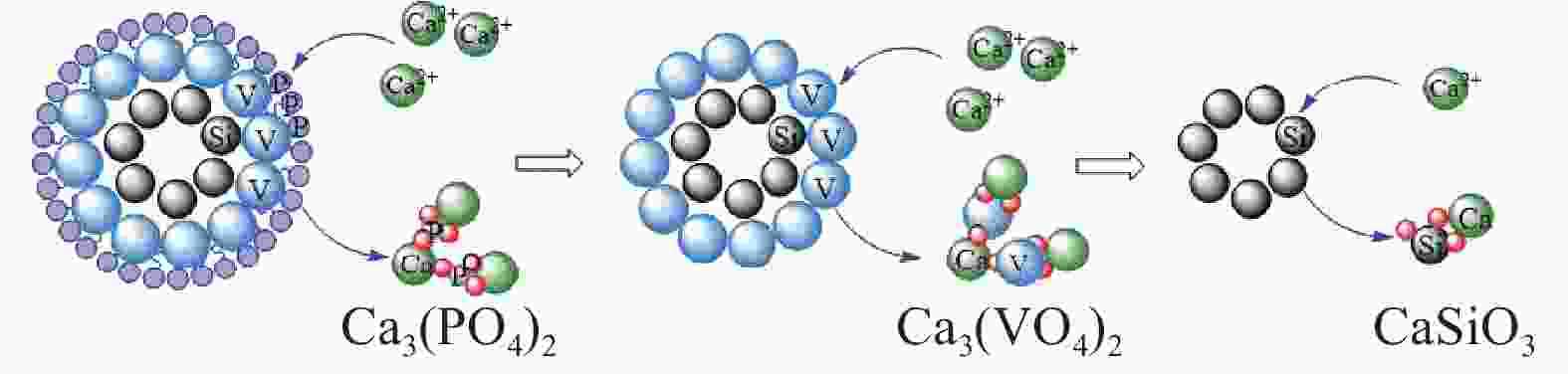
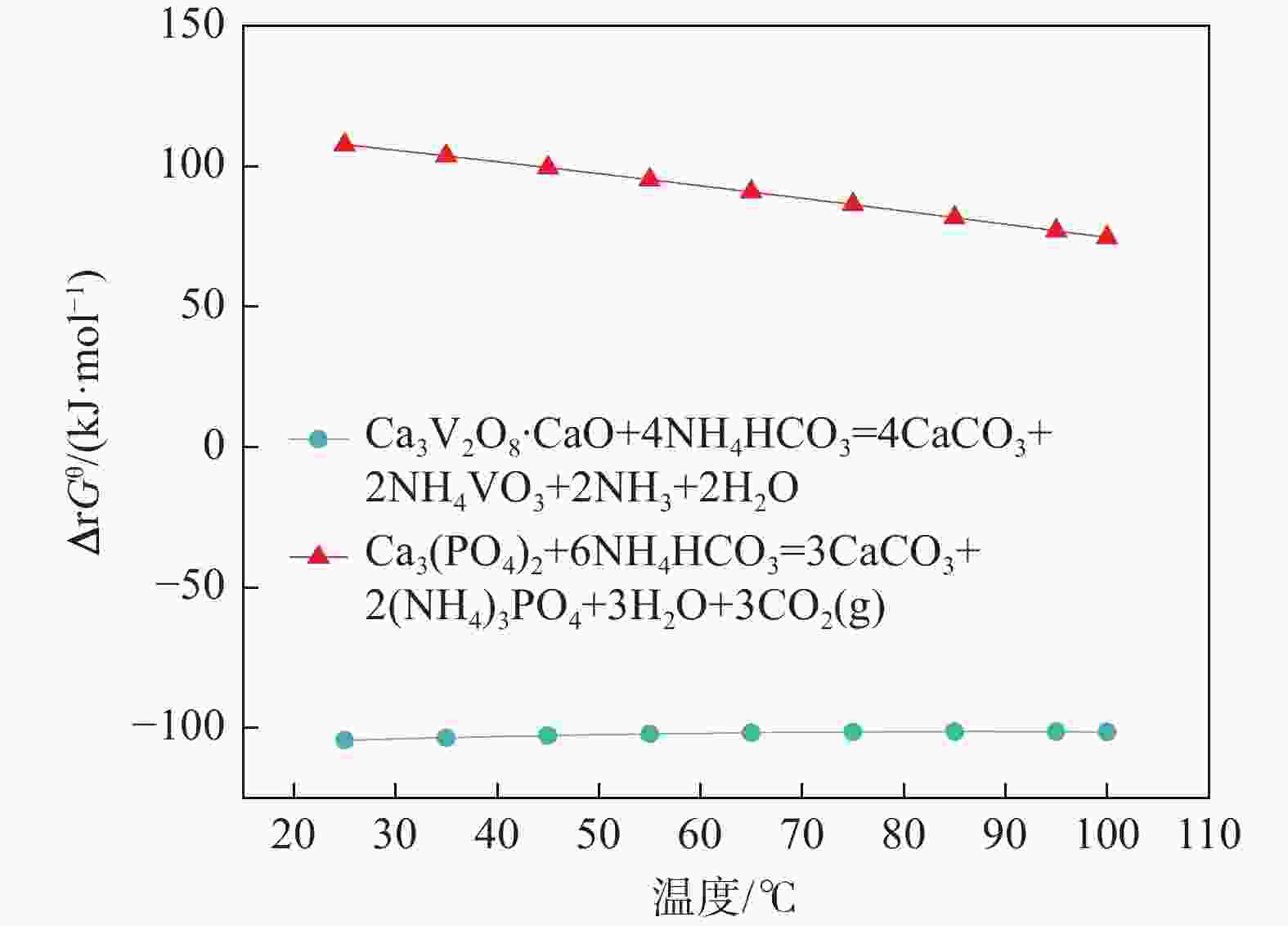
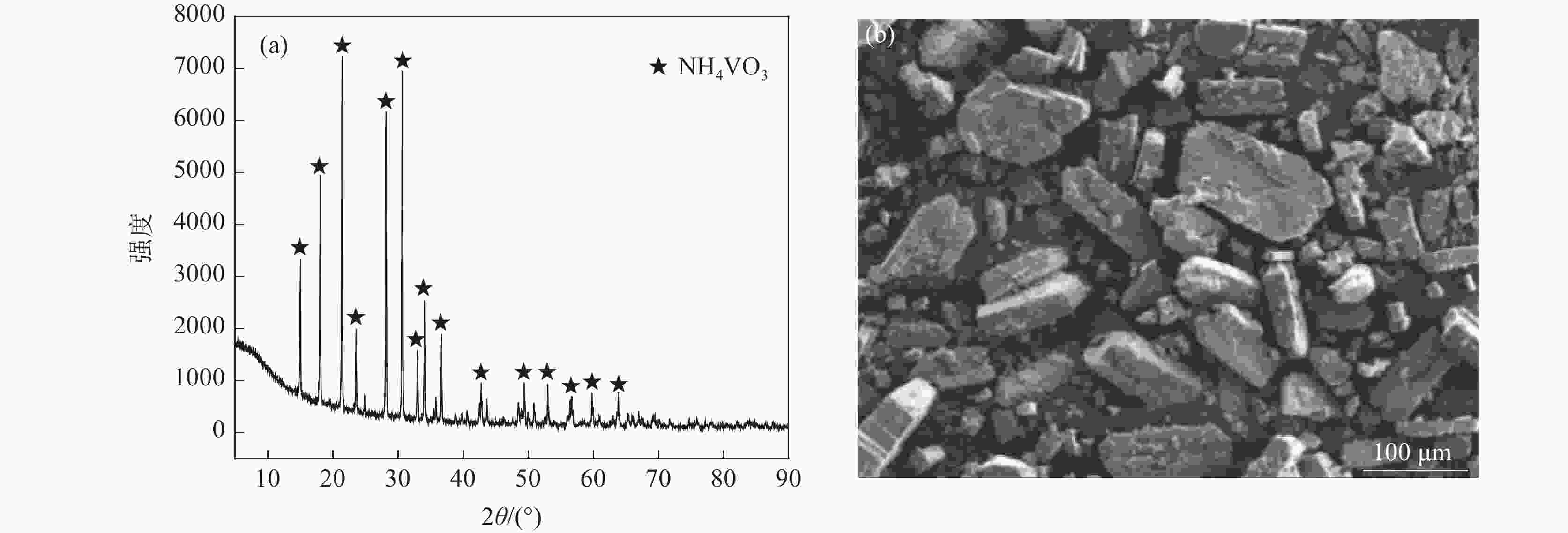
成分 含量/(g·L−1) 含量/% NaOH浸出液 钙化后碱液 铵化后液 钒酸钙 碳酸钙 Na2O 34.95 34.82 0.02 SiO2 0.39 0.32 0.06 0.17 0.99 Al2O3 0.58 0.55 P2O5 3.11 0.28 0.18 8.34 6.85 V2O5 16.22 0.52 29.45 40.38 1.55 -
[1] Hou Shuai, Tian Ying, Li Yungang. Research progress in preparation methods of vanadium metal[J]. Rare Metals and Cemented Carbides, 2022,50(6):22-26. (侯帅, 田颖, 李运刚. 金属钒制备方法的研究进展[J]. 稀有金属与硬质合金, 2022,50(6):22-26.Hou Shuai, Tian Ying, Li Yungang. Research progress in preparation methods of vanadium metal[J]. Rare Metals and Cemented Carbides, 2022, 50(6): 22-26. [2] Zeng Lingyun. Analysis and suggestions on the mining situation of vanadium resources in China[J]. Mineral Resources, 2020(16):80-81. (曾凌云. 我国钒矿资源开采形势分析与建议[J]. 世界有色金属, 2020(16):80-81.Zeng Lingyun. Analysis and suggestions on the mining situation of vanadium resources in China[J]. Mineral Resources, 2020(16): 80-81. [3] Sun Ying, Zhang Ting,an, Lü Guozhi, et al. Separation of vanadium and iron from vanadium-bearing acidic solution by anion extraction[J]. Non-ferrous Metals (Smelting Part), 2021(4):41-47. (孙颖, 张廷安, 吕国志, 等. 含钒酸性溶液阴离子萃取分离钒铁的研究[J]. 有色金属(冶炼部分), 2021(4):41-47.Sun Ying, Zhang Ting, an, Lü Guozhi, et al. Separation of vanadium and iron from vanadium-bearing acidic solution by anion extraction[J]. Non-ferrous Metals (Smelting Part), 2021(4): 41-47. [4] Chen Bingxu, Du Hao, Li Lanjie, et al. Research progress on resource utilization on deactivated sulfuric acid catalyst[J]. Hebei Metallurgy, 2022(10):1-8, 20. (陈炳旭, 杜浩, 李兰杰, 等. 失活硫酸催化剂资源化利用研究进展[J]. 河北冶金, 2022(10):1-8, 20.Chen Bingxu, Du Hao, Li Lanjie, et al. Research progress on resource utilization on deactivated sulfuric acid catalyst[J]. Hebei Metallurgy, 2022(10): 1-8, 20. [5] Erust C, Akcil A, Bedelova Z, et al. Recovery of vanadium from spent catalysts of sulfuric acid plant by using inorganic and organic acids: Laboratory and semi-pilot tests[J]. Waste Management, 2016,49:455-461. doi: 10.1016/j.wasman.2015.12.002 [6] Khorfan S, Wahoud A, Reda Y. Recovery of vanadium pentoxide from spent catalyst used in the manufacture of sulphuric acid[J]. Periodica Polytechnica Chemical Engineering, 2001,45(2):131-137. [7] Wahoud A, Alouche A, Abdulbake M. Sulfuric acid baking and leaching of spent sulfuric acid catalyst[J]. Periodica Polytechnica Chemical Engineering, 2011,55(1):31-34. doi: 10.3311/pp.ch.2011-1.06 [8] Zhao Beibei, Li Lanjie, Liu Lin, et al. Study on vanadium extraction process of spent vanadium catalyst[J]. Multipurpose Utilization of Mineral Resources, 2019(6):80-83. (赵备备, 李兰杰, 柳林, 等. 废钒触媒提钒工艺研究[J]. 矿产综合利用, 2019(6):80-83.Zhao Beibei, Li Lanjie, Liu Lin, et al. Study on vanadium extraction process of spent vanadium catalyst[J]. Multipurpose Utilization of Mineral Resources, 2019(6): 80-83. [9] Mousa K M, Kouba S K. Study on vanadium recovery from spent catalyst used in the manufacture of sulfuric acid[J]. Iraqi Journal of Chemical and Petroleum Engineering, 2010,11(2):49-54. doi: 10.31699/IJCPE.2010.2.6 [10] Mazurek K, Grzesiak P, Druyński S, et al. Method of utilization of the spent vanadium catalyst[J]. Polish Journal of Chemical Technology, 2018, 20(3):31. [11] Romanovskaia E, Romanovski V, Kwapinski W, et al. Selective recovery of vanadium pentoxide from spent catalysts of sulfuric acid production: Sustainable approach[J]. Hydrometallurgy, 2021(200): 200. [12] Dongseo L, Sung Ho J, Shin D, et al. Recovery of vanadium and cesium from spent sulfuric acid catalysts by a hydrometallurgical process [J]. Green Chemistry, 2022, 24(2): 790-799. [13] Hu Jianfeng. Study on new technology for extracting vanadium from waste vanadium catalyst[D]. Kunming: Kunming University of Science and Technology, 2006. (胡建锋. 从废钒触媒中提钒新工艺的研究[D]. 昆明: 昆明理工大学, 2006.Hu Jianfeng. Study on new technology for extracting vanadium from waste vanadium catalyst[D]. Kunming: Kunming University of Science and Technology, 2006. [14] Jin Xiuju. Vanadium extraction from stone coal by oxidizing roasting-compound alkaline leaching method[D]. Changsha: Hunan University, 2015. (金秀举. 石煤钒矿氧化焙烧—复合碱浸提钒工艺研究[D]. 长沙: 湖南大学, 2015.Jin Xiuju. Vanadium extraction from stone coal by oxidizing roasting-compound alkaline leaching method[D]. Changsha: Hunan University, 2015. [15] Ke Zhaohua, Li Qinggang, Zeng Chengwei. Vanadium extraction from carbonaceous shale by blank roasting-pressurized high temperature alkali leaching[J]. Rare Metals and Cemented Carbides, 2011,39(2):10-13. (柯兆华, 李青刚, 曾成威. 空白焙烧-加压高温碱浸法从石煤中提钒的试验研究[J]. 稀有金属与硬质合金, 2011,39(2):10-13.Ke Zhaohua, Li Qinggang, Zeng Chengwei. Vanadium extraction from carbonaceous shale by blank roasting-pressurized high temperature alkali leaching[J]. Rare Metals and Cemented Carbides, 2011, 39(2): 10-13. [16] Wang Shaona, Du Hao, Zheng Shili, et al. New technology from sodium vanadate oxide by calcification and carbonization-ammonium process[J]. CIESC, 2017,68(7):2781-2789. (王少娜, 杜浩, 郑诗礼, 等. 钒酸钠钙化-碳化铵沉法清洁制备钒氧化物新工艺[J]. 化工学报, 2017,68(7):2781-2789. doi: 10.11949/j.issn.0438-1157.20161511Wang Shaona, Du Hao, Zheng Shili, et al. New technology from sodium vanadate oxide by calcification and carbonization-ammonium process[J]. CIESC, 2017, 68(7): 2781-2789. doi: 10.11949/j.issn.0438-1157.20161511 -





 下载:
下载: