Preparation of VN by reduction nitriding V2O3 with CH4
-
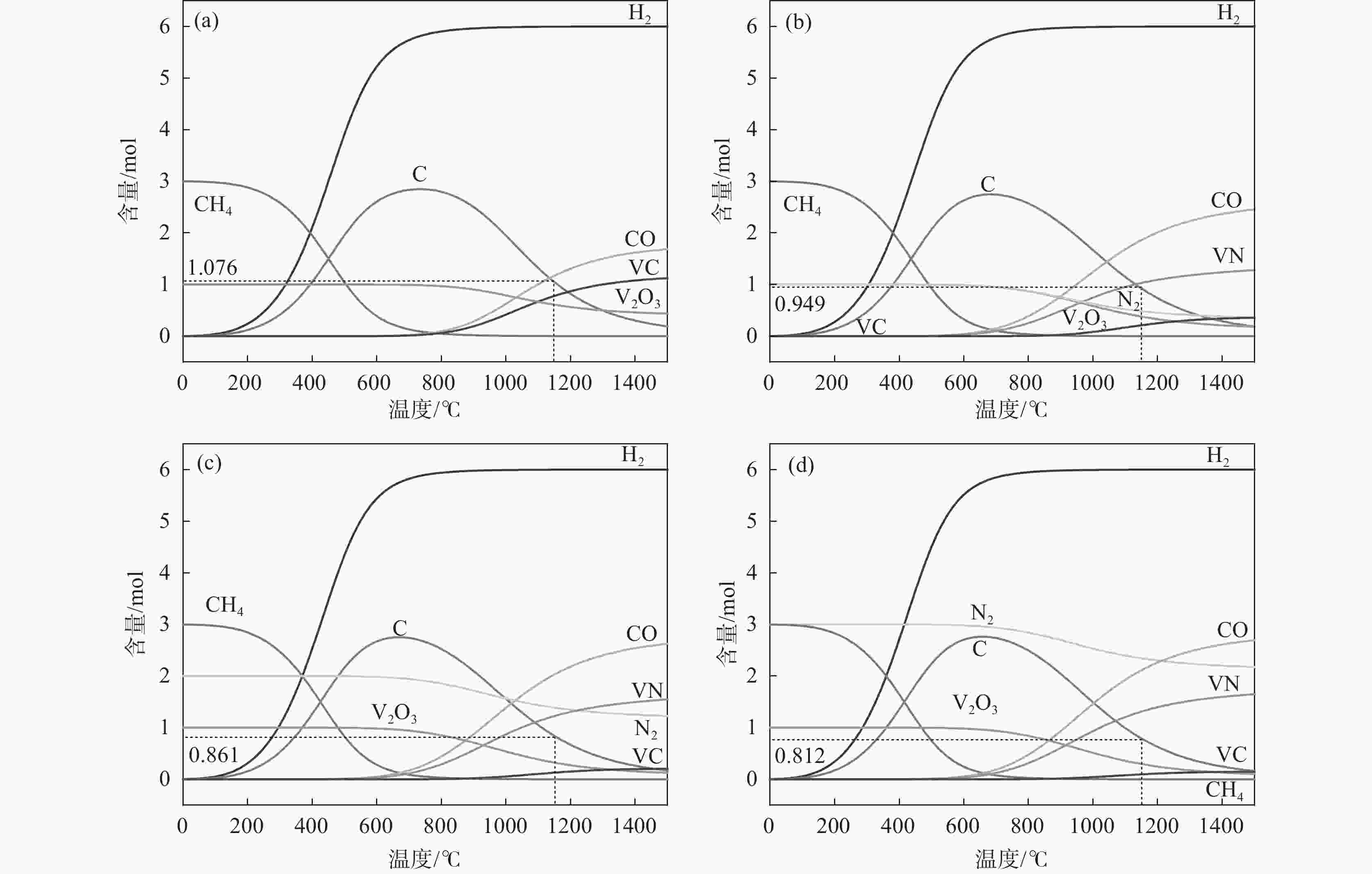
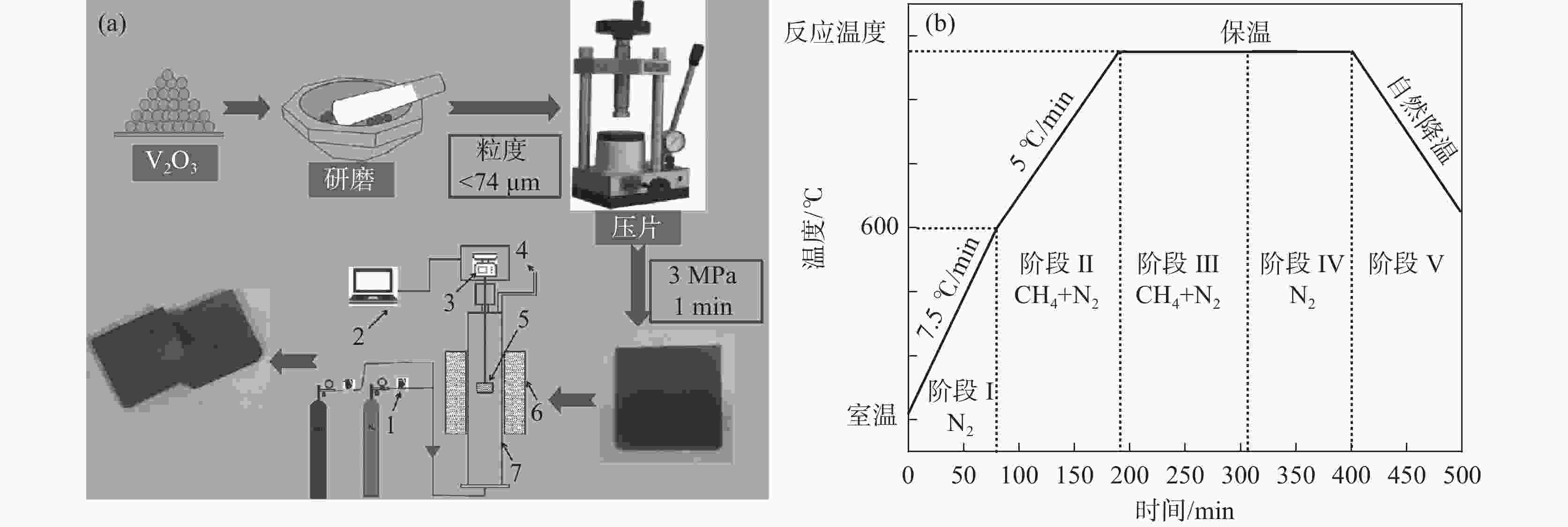
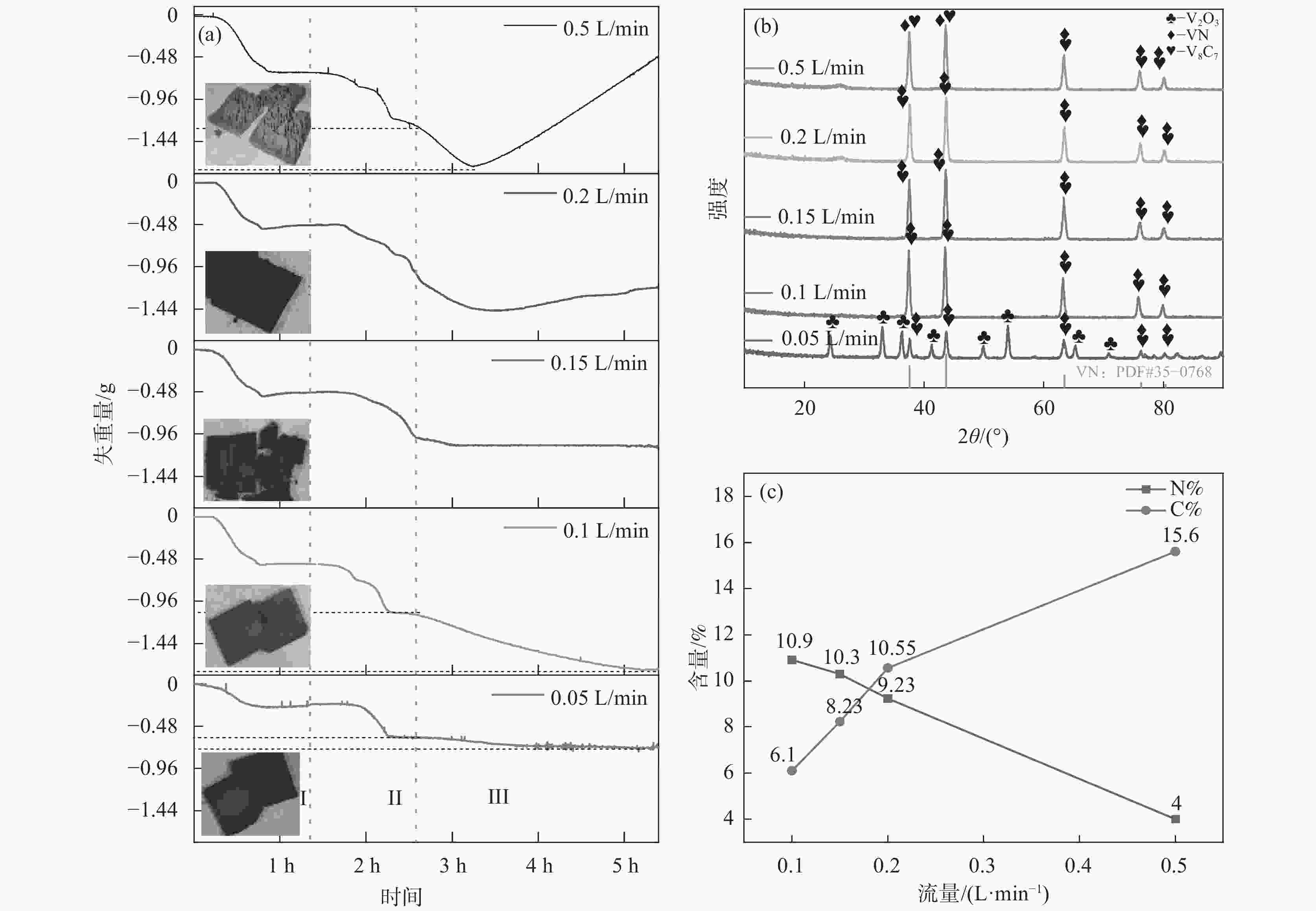
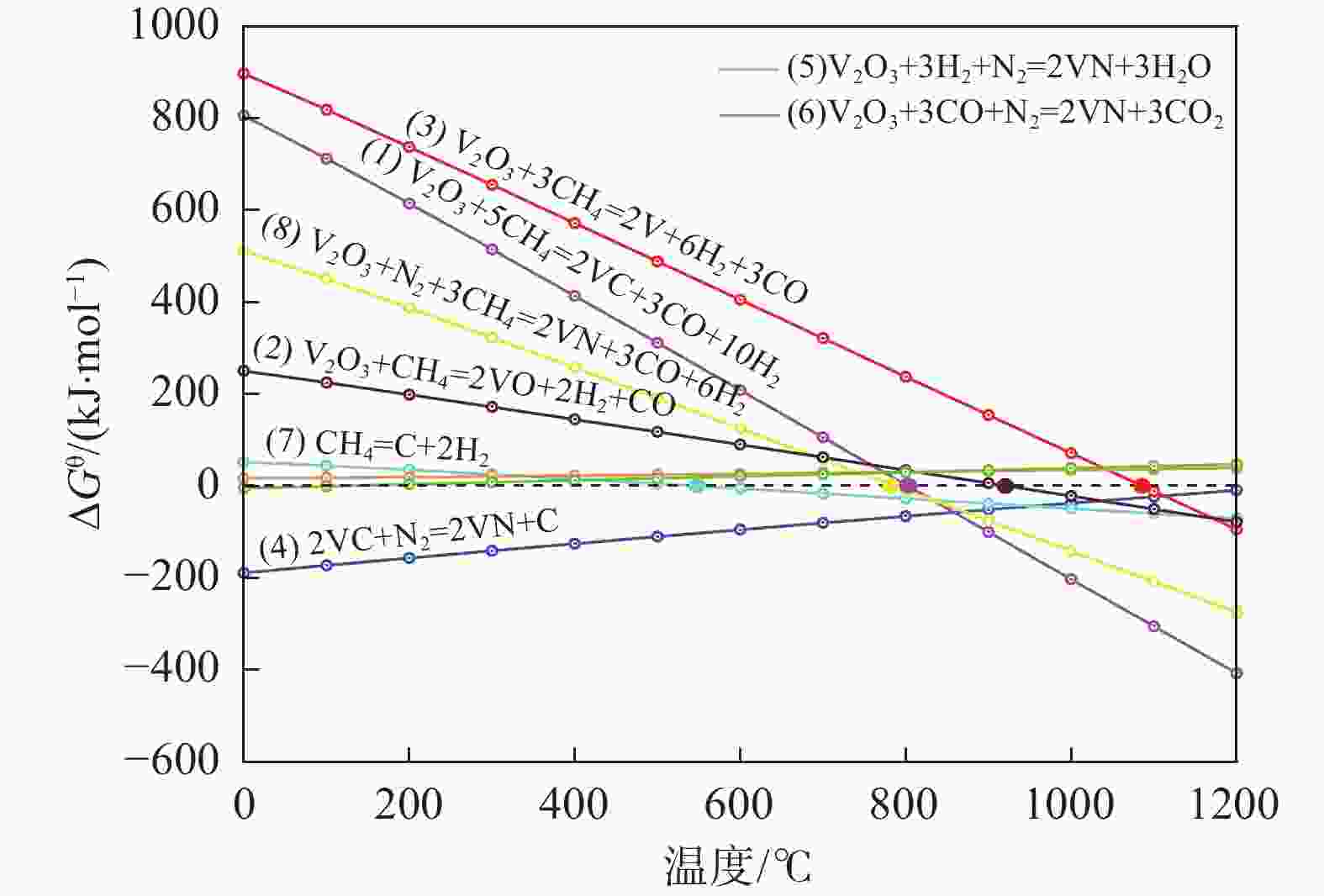
摘要: 通过热力学计算和试验工艺结合的方式,探究了利用CH4作为还原剂碳热还原、氮化制备VN的工艺条件以及反应过程。结果表明:在CH4流量0.1 L/min、
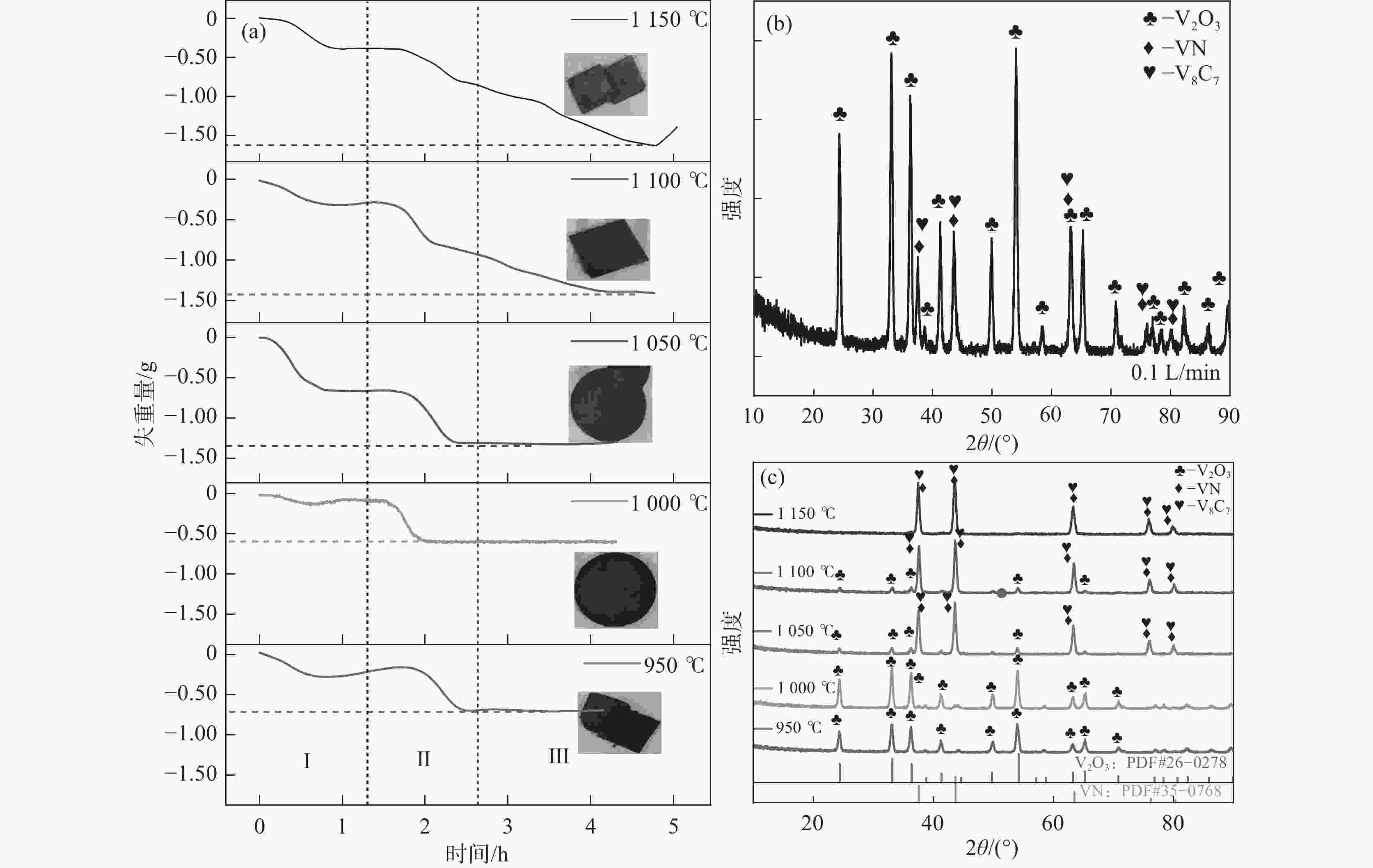
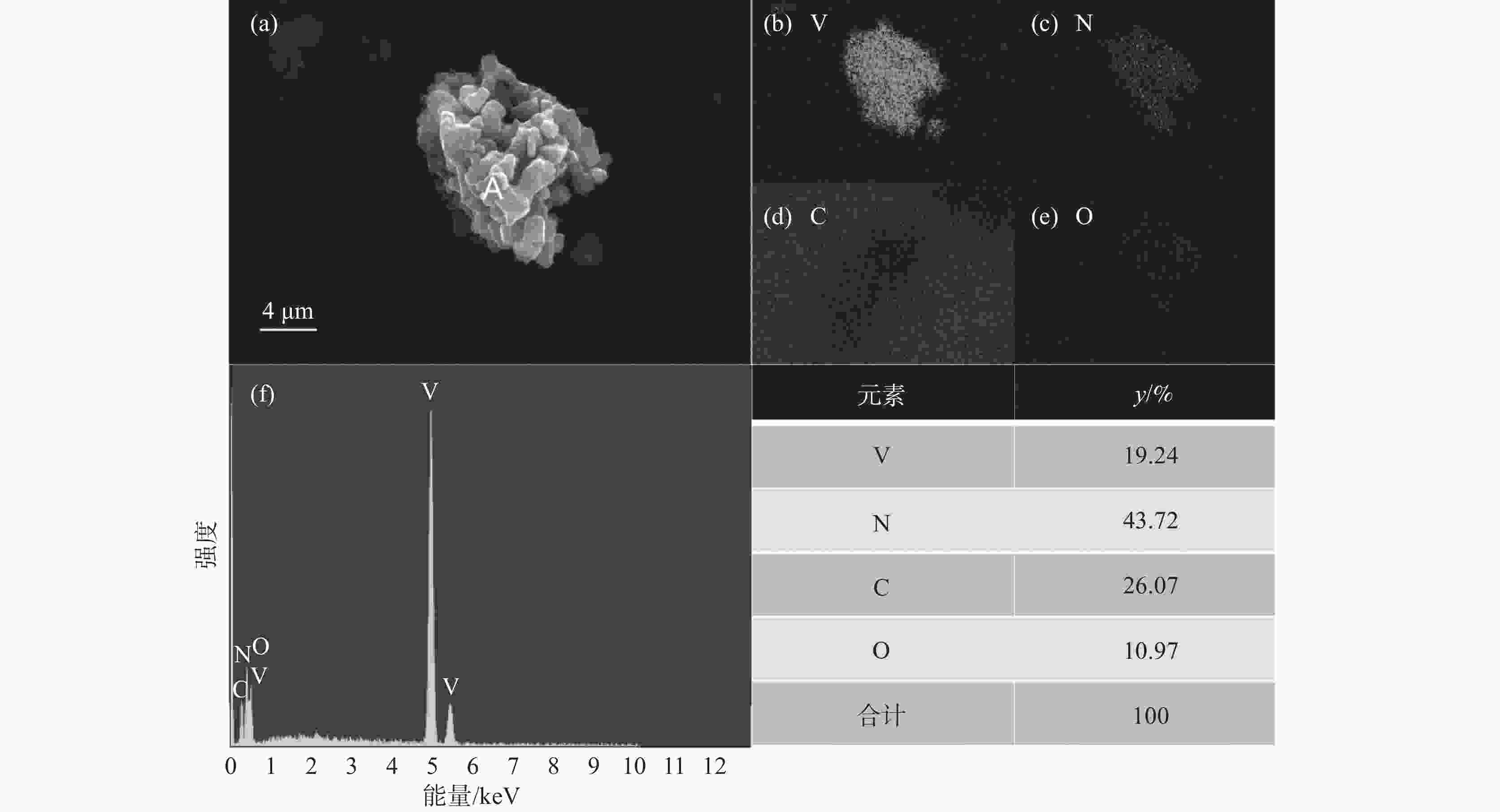
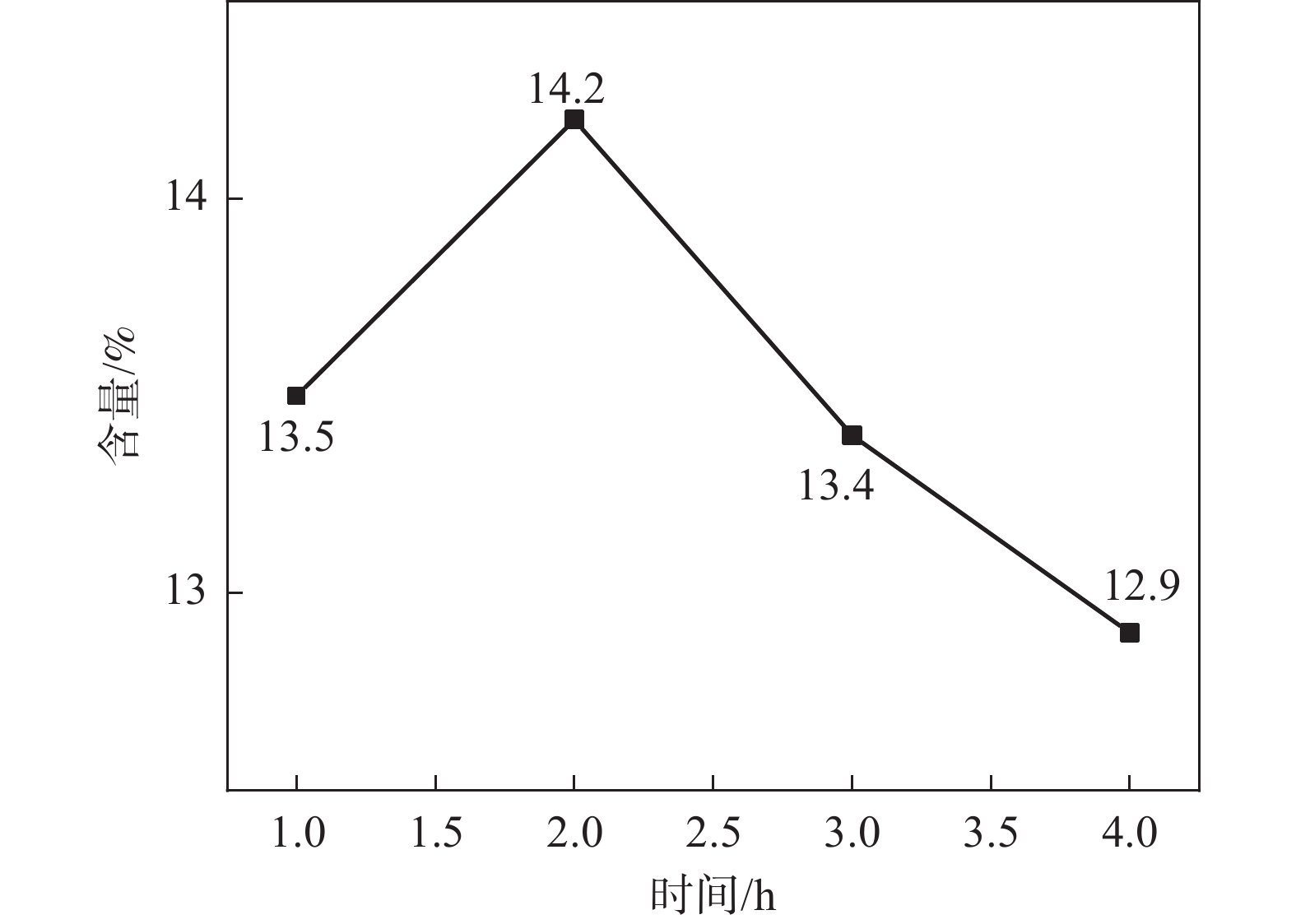
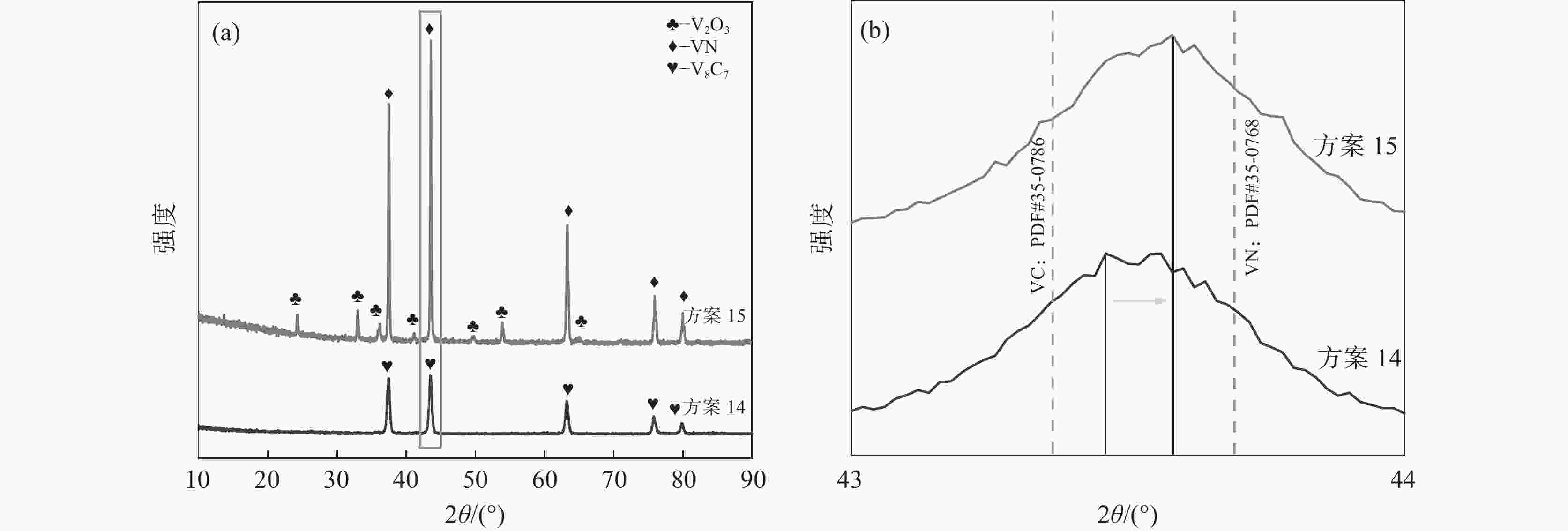
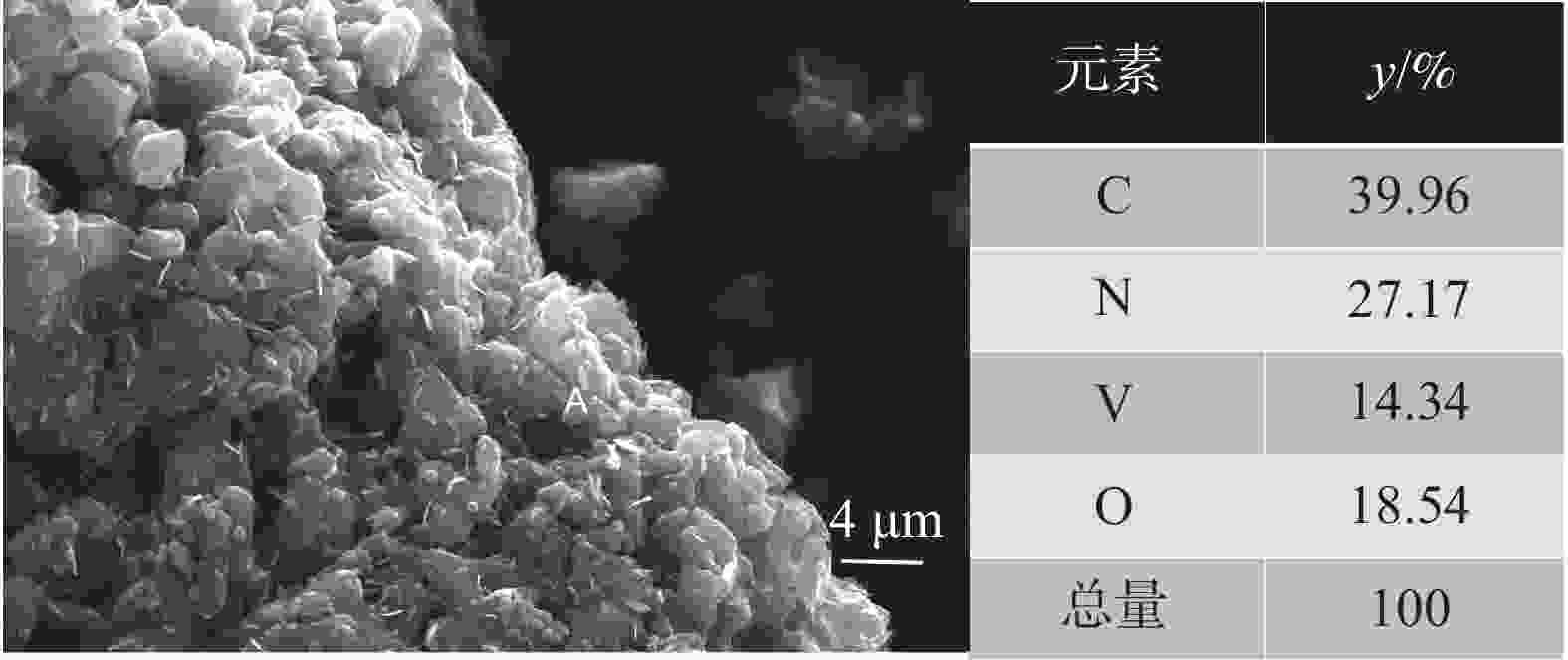
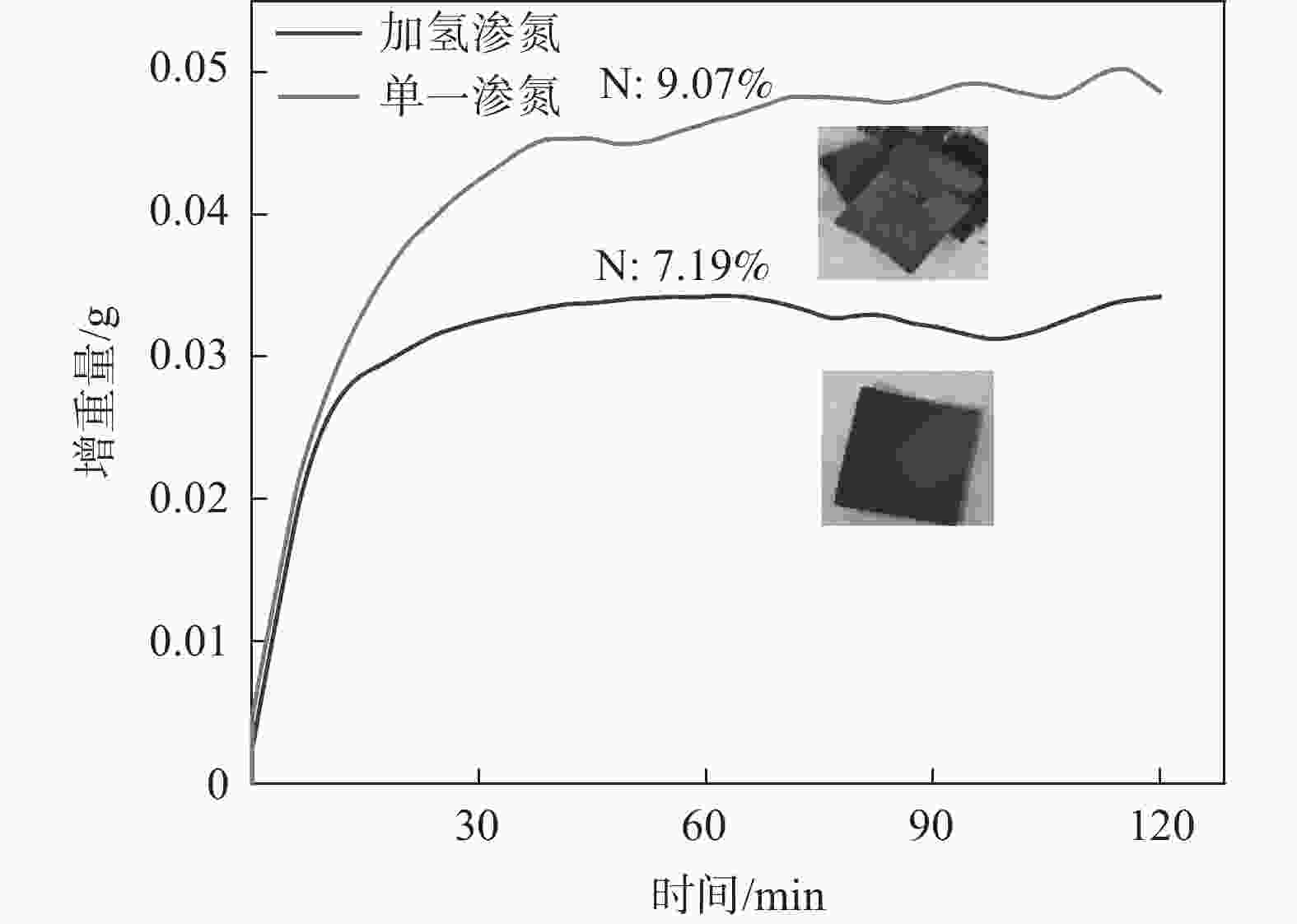
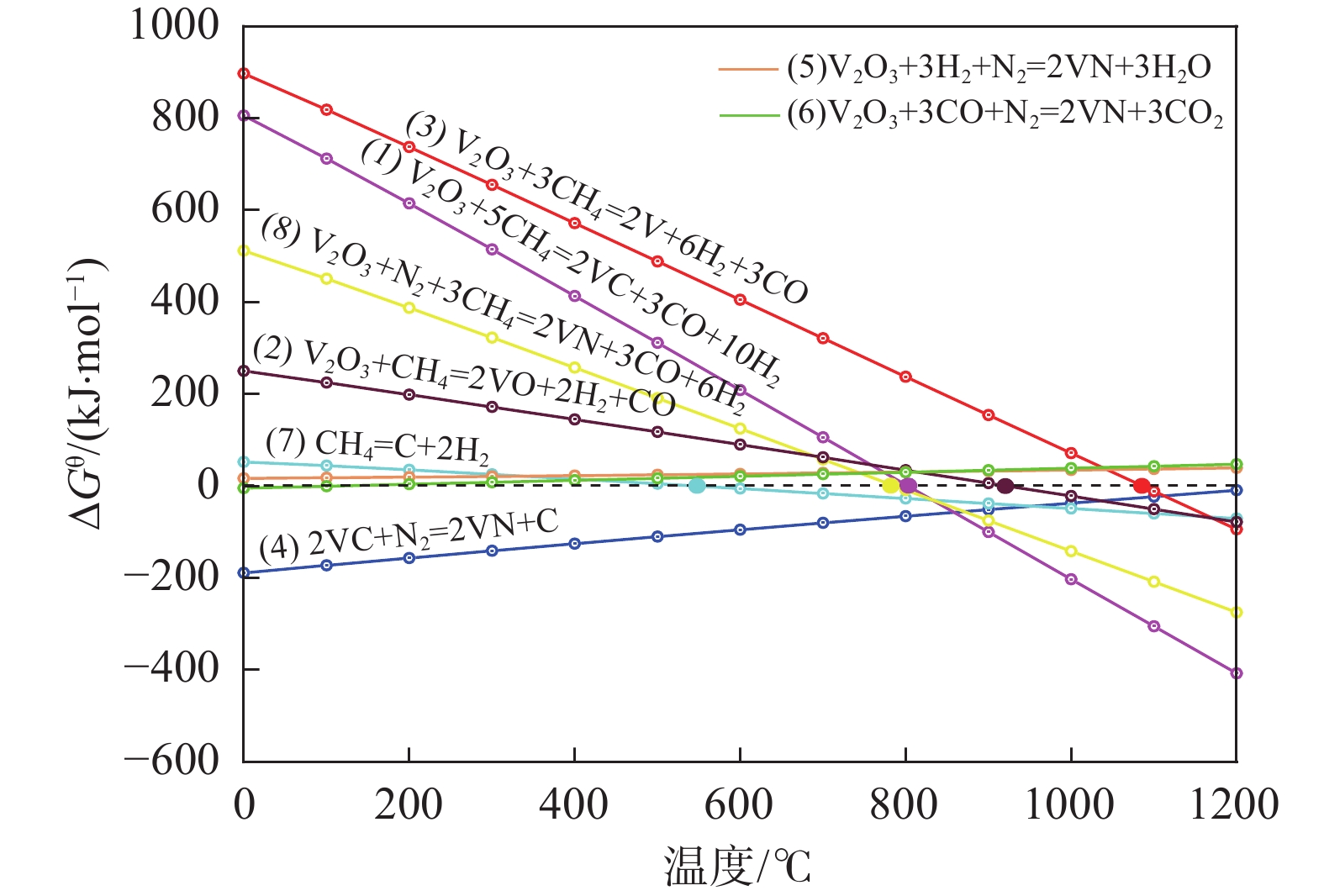
1150 ℃保温2 h并继续渗氮2 h的条件下,成功合成了氮、碳含量分别为14.2%、3.35%的VN产物,符合国家VN16(GB/T20567 -2020)氮化钒产品标准。反应过程中的物相转变过程为V2O3→V8C7→VN,并且CH4在高温条件下分解产生的高活性炭与H2均有利于碳化反应的进行,能有效提高反应速率。Abstract: In this paper, the process conditions and reaction process of using CH4 instead of traditional carbon thermal reduction to prepare VN is explored by combining thermodynamic calculation and experimental process exploration. The results showed that the VN products with nitrogen and carbon contents of 14.2% and 3.35% were successfully synthesized under the conditions of CH4 flow rate of 0.1 L/min, heating at1150 ℃ for 2 h and continuous nitriding for 2 h, which met the national standard of VN16 (GB/T 20567-2020) for vanadium nitride product. The phase transition process during the reaction is V2O3→V8C7→VN, and the high activated carbon and hydrogen produced by methane decomposition at high temperature are beneficial to the carbonization reaction and effectively improve the reaction rate.-
Key words:
- VN /
- V2O3 /
- CH4 /
- reduction nitridation /
- phase transitions
-
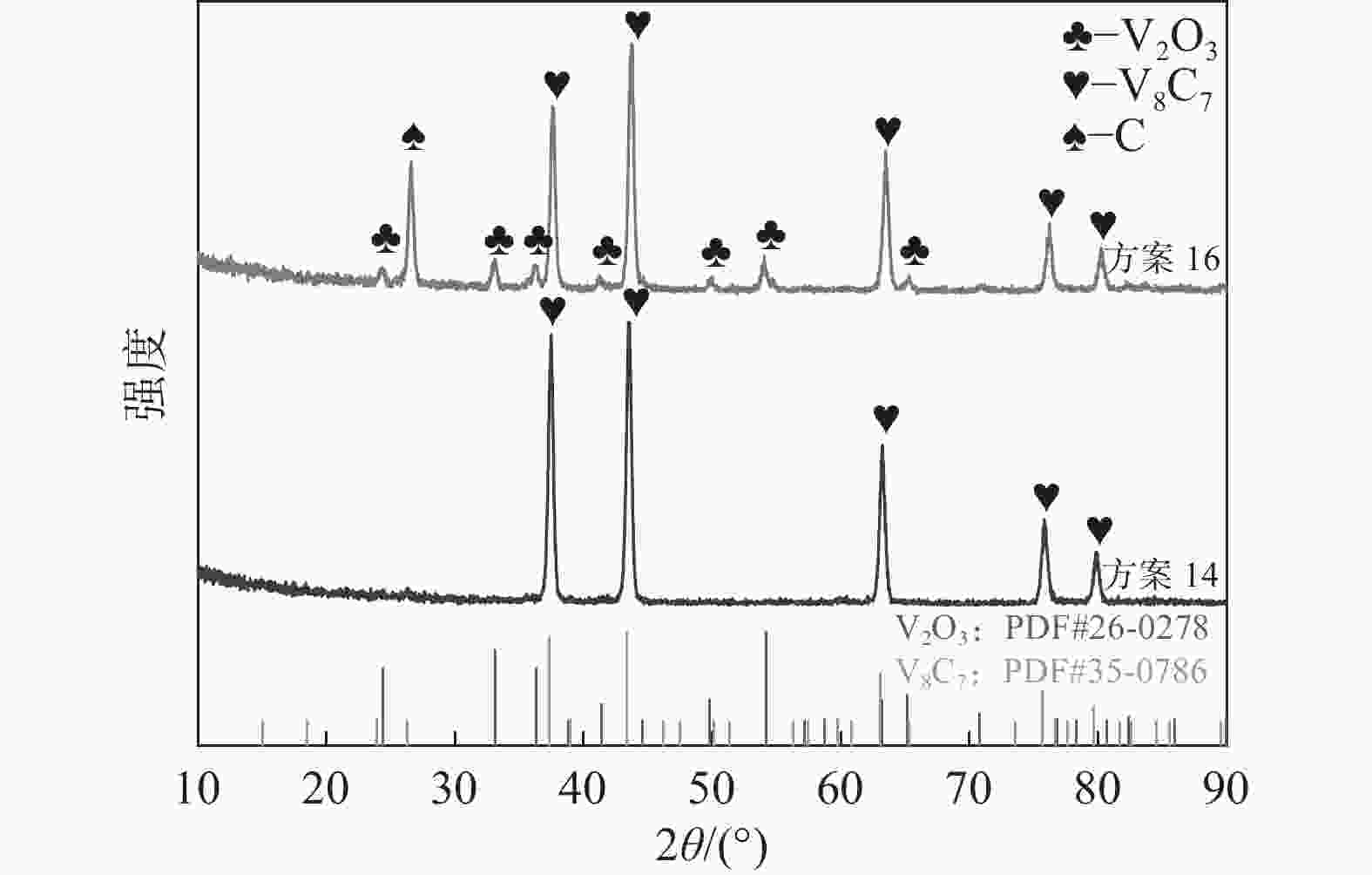
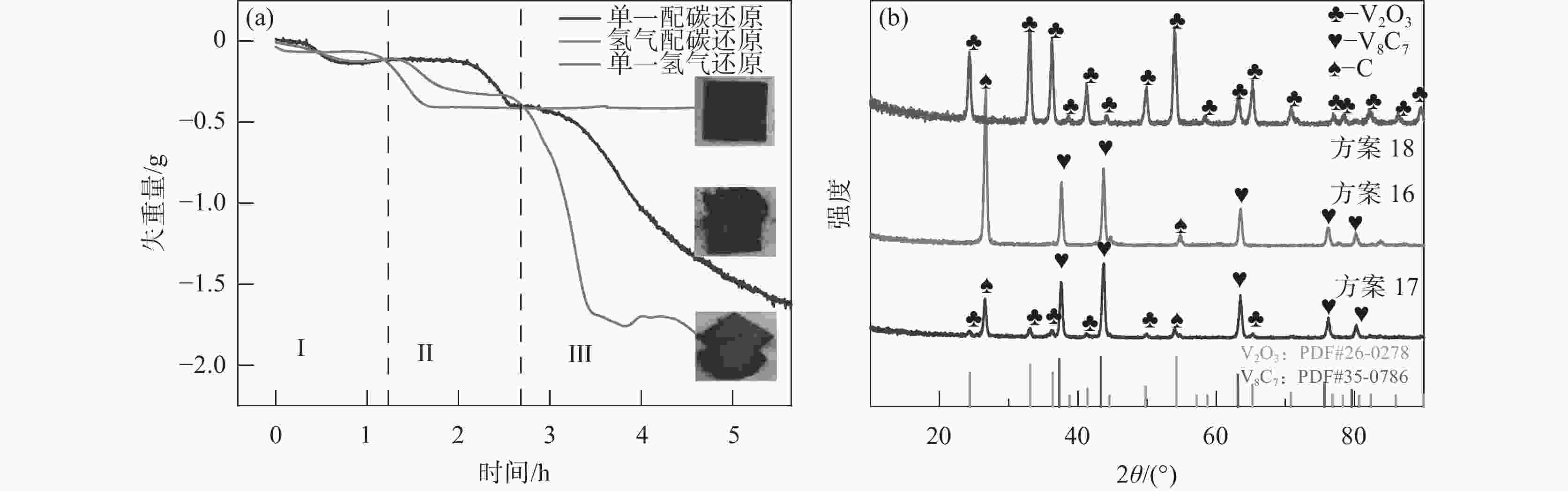
表 1 试验方案
Table 1. Experimental scheme
方案 反应物 气氛组成 甲烷流量
/(L·min−1)温度/℃ 保温时间/h 渗氮时间/h 1 V2O3 CH4+N2 0.1 950 2 0 2 V2O3 CH4+N2 0.1 1000 2 0 3 V2O3 CH4+N2 0.1 1050 2 0 4 V2O3 CH4+N2 0.1 1100 2 0 5 V2O3 CH4+N2 0.1 1150 2 0 6 V2O3 CH4+N2 0.15 1150 2 0 7 V2O3 CH4+N2 0.2 1150 2 0 8 V2O3 CH4+N2 0.25 1150 2 0 9 V2O3 CH4+N2 0.05 1150 2 0 10 V2O3 CH4+N2 0.1 1150 2 1 11 V2O3 CH4+N2 0.1 1150 2 2 12 V2O3 CH4+N2 0.1 1150 2 3 13 V2O3 CH4+N2 0.1 1150 2 4 14 V2O3 CH4 0.1 1150 2 0 15 VC N2 1150 2 0 16 V2O3+C H2 1150 2 17 V2O3+C 1150 2 18 V2O3 H2 1150 2 19 VC N2 1150 2 20 VC N2+H2 1150 2 -
[1] Zhou Zhenyu. Study on the basis and application of deep vanadium extraction and carbon preservation in vanadium-bearing molten iron combined blowing converter [D]. Chongqing: Chongqing University, 2019. (周振宇. 含钒铁水复吹转炉深提钒和保碳的基础及应用研究[D]. 重庆: 重庆大学, 2019.Zhou Zhenyu. Study on the basis and application of deep vanadium extraction and carbon preservation in vanadium-bearing molten iron combined blowing converter [D]. Chongqing: Chongqing University, 2019. [2] Tan Ruobin. Development and application of vanadium, vanadium compounds and vanadium alloys[J]. Vanadium and Titanium, 1995(1):1-10. (谭若斌. 钒、钒化合物、钒合金的开发应用[J]. 钒钛, 1995(1):1-10.Tan Ruobin. Development and application of vanadium, vanadium compounds and vanadium alloys[J]. Vanadium and Titanium, 1995(1): 1-10. [3] Duan Xinhui, Srinivasakannan C, Zhang H, et al. Process optimization of the preparation of vanadium nitride from vanadium pentoxide[J]. Arabian Journal for Science and Engineering, 2015, 40(8): 2133-2139. [4] Xu Rui, Wu Yuedong, Zhang Guohua. Preparation of high purity vanadium nitride by magnesiothermic reduction of V2O3 followed by nitriding in N2 atmosphere[J]. Transactions of Nonferrous Metals Society of China, 2019, 29(8): 1776-1783. (徐瑞, 吴跃东, 张国华. 用镁热还原法制备高纯氮化钒[J]. 中国有色金属学会学报, 2019, 29(8): 1776-1783.Xu Rui, Wu Yuedong, Zhang Guohua. Preparation of high purity vanadium nitride by magnesiothermic reduction of V2O3 followed by nitriding in N2 atmosphere[J]. Transactions of Nonferrous Metals Society of China, 2019, 29(8): 1776-1783. [5] Mechnik V A, Bondarenko N A, Kuzin N O. Influence of the addition of vanadium nitride on the structure and specifications of a diamond–(Fe–Cu–Ni–Sn) composite system[J]. Frict. Wear, 2018, 39 (2) : 108-113. [6] Wu Yuedong, Zhang Guohua, Zhou Guozhi. A novel process to synthesize high-quality ferrovanadium nitride[J]. Metallurgical and Materials Transactions, 2016,47(6):3405-3412. (吴跃东, 张国华, 周国治. 合成高质量氮化钒铁的新工艺[J]. 冶金材料学报, 2016,47(6):3405-3412. doi: 10.1007/s11663-016-0793-8Wu Yuedong, Zhang Guohua, Zhou Guozhi. A novel process to synthesize high-quality ferrovanadium nitride[J]. Metallurgical and Materials Transactions, 2016, 47(6): 3405-3412. doi: 10.1007/s11663-016-0793-8 [7] Zhong Yu, Chao Dongliang, Deng Shengjue, et al. Confining sulfur in integrated composite scaffold with highly porous carbon fibers/vanadium nitride arrays for high-performance lithium-sulfur batteries[J]. Advanced Functional Materials, 2018,28(38):1706391. (钟宇, 晁栋梁, 邓胜珏, 等. 高性能锂硫电池用多孔碳纤维/氮化钒阵列集成复合支架中的硫限制[J]. 先进功能材料, 2018,28(38):1706391. doi: 10.1002/adfm.201706391Zhong Yu, Chao Dongliang, Deng Shengjue, et al. Confining sulfur in integrated composite scaffold with highly porous carbon fibers/vanadium nitride arrays for high-performance lithium-sulfur batteries[J]. Advanced Functional Materials, 2018, 28(38): 1706391. doi: 10.1002/adfm.201706391 [8] Tao Jun, Tao Ran, Yan Baijun. Experimental measurements of activity coefficients of vanadium in liquid iron[J]. Nonferrous Metals Science and Engineering, 2017,8(1):11-14. (陶俊, 陶然, 闫柏军. 钒在铁液中活度系数的实验研究[J]. 有色金属科学与工程, 2017,8(1):11-14.Tao Jun, Tao Ran, Yan Baijun. Experimental measurements of activity coefficients of vanadium in liquid iron[J]. Nonferrous Metals Science and Engineering, 2017, 8(1): 11-14. [9] Bondarchuk O, Morel A, Bélanger D, et al. Thin films of pure vanadium nitride: Evidence for anomalous non–faradaic capacitance[J]. Journal of Power Sources, 2016,324(5):439-446. [10] Wang Baohua, Wu Chunliang, Lu Yongjie, et al. Study on industrial production of high-nitrogen content VN-alloy[J]. Nonferrous Metals, 2019(7):64-67. (王宝华, 吴春亮, 卢永杰, 等. 工业生产高氮氮化钒合金的研究[J]. 有色金属(冶炼部分), 2019(7):64-67.Wang Baohua, Wu Chunliang, Lu Yongjie, et al. Study on industrial production of high-nitrogen content VN-alloy[J]. Nonferrous Metals, 2019(7): 64-67. [11] Chu Zhiqiang, Guo Xueyi, Tian Qinghua, et al. Preparation of vanadium nitride by carbothermic reduction and nitrogenization[J]. Materials Science and Engineering of Powder Metallurgy, 2015,20(6):965-970. (储志强, 郭学益, 田庆华, 等. 碳热还原氮化法制备氮化钒[J]. 粉末冶金材料科学与工程, 2015,20(6):965-970. doi: 10.3969/j.issn.1673-0224.2015.06.022Chu Zhiqiang, Guo Xueyi, Tian Qinghua, et al. Preparation of vanadium nitride by carbothermic reduction and nitrogenization[J]. Materials Science and Engineering of Powder Metallurgy, 2015, 20(6): 965-970. doi: 10.3969/j.issn.1673-0224.2015.06.022 [12] Yin Qi. Mechanism study on preparation of vanadium nitride by carbothermal reduction nitridation method[D]. Kunming: Kunming University of Science and Technology, 2023. (尹奇. 碳热还原氮化法制备氮化钒的机理研究[D]. 昆明: 昆明理工大学, 2023.Yin Qi. Mechanism study on preparation of vanadium nitride by carbothermal reduction nitridation method[D]. Kunming: Kunming University of Science and Technology, 2023. [13] Zhao Shiqiang. Study on the preparation of V(N, O) powder by reduction of V2O5 with ammonia at low temperatures[J]. Nonferrous Metals Science and Engineering, 2019,10(5):28-34. (赵世强. 氨气还原氮化五氧化二钒制备V(N, O)粉体与机理研究[J]. 有色金属科学与工程, 2019,10(5):28-34.Zhao Shiqiang. Study on the preparation of V(N, O) powder by reduction of V2O5 with ammonia at low temperatures[J]. Nonferrous Metals Science and Engineering, 2019, 10(5): 28-34. [14] Fu Mingkai, Jin Jiahui, Ma Haitao, et al. Thermodynamic analysis of novel vanadium redox materials for solar thermochemical ammonia synthesis from N2 and CH4[J]. International Journal of Hydrogen Energy, 2020,45(4):2569-2577. doi: 10.1016/j.ijhydene.2019.11.122 [15] Rodrı́guez P, Brito J L, Albornoz A, et al. Comparison of vanadium carbide and nitride catalysts for hydrotreating[J]. Catalysis Communications, 2004,5(2):79-82. doi: 10.1016/j.catcom.2003.11.011 [16] Choi Jeong Gil, Ha Joseph, Hong Jin Who. Synthesis and catalytic properties of vanadium interstitial compounds[J]. Applied Catalysis A: General, 1998,168(1):47-56. doi: 10.1016/S0926-860X(97)00332-3 [17] Gao Feng. Fundamental study on preparing vanadium nitride and carbide by carbothermal reduction and nitridation of V2O3[D]. Shenyang: Northeastern University, 2006. (高锋. 三氧化二钒碳热还原氮化制备碳氮化钒的基础研究[D]. 沈阳: 东北大学, 2006.Gao Feng. Fundamental study on preparing vanadium nitride and carbide by carbothermal reduction and nitridation of V2O3[D]. Shenyang: Northeastern University, 2006. -




 下载:
下载: