Phase coexistence relationship of CaO-Al2O3-V2O5 slag system
-
摘要: CaO-Al2O3-V2O5渣系是钢液脱氮渣系的基础氧化物渣系,其相平衡关系等热力学信息对于相关渣系的研究和开发至关重要。通过高温相平衡试验,结合扫描电镜和能谱分析,研究了CaO-Al2O3-V2O5渣系的相平衡关系。根据试验结果,确定了CaO-Al2O3-V2O5渣系
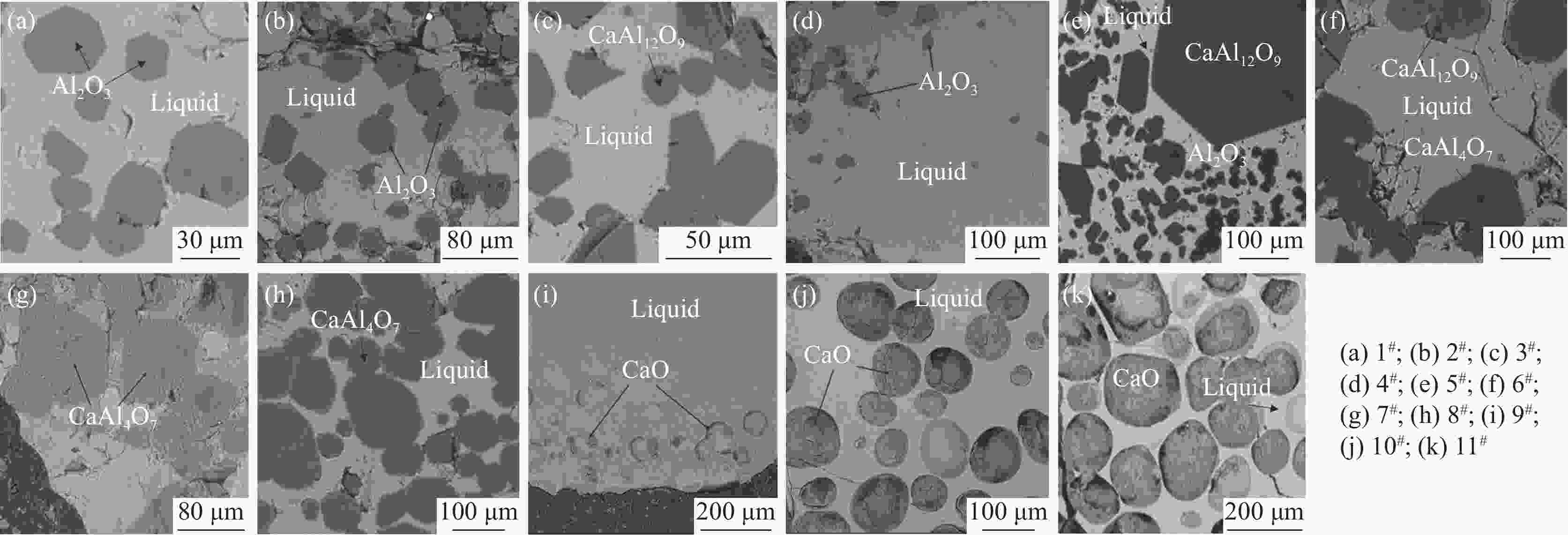
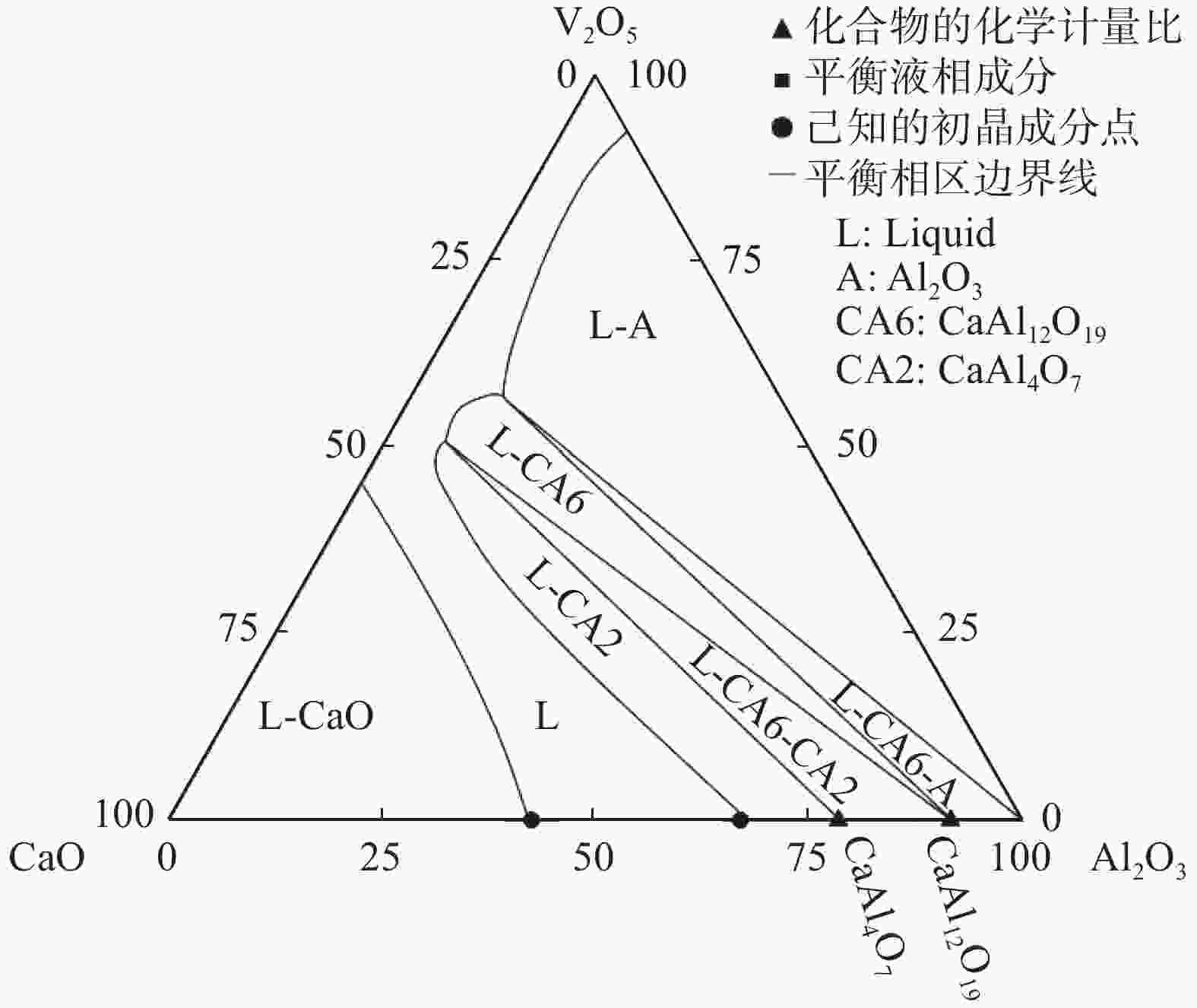
1 600 ℃空气气氛条件下的相平衡关系,并绘制该体系全成分范围内CaO-Al2O3-V2O5渣系的等温截面图,共包含7个平衡相区,分别是:Liquid+Al2O3+CaAl12O19、Liquid+CaAl12O19+CaAl4O7、Liquid+CaO、Liquid+Al2O3、Liquid+CaAl12O19、Liquid+CaAl4O7和单一液相区。上述平衡相区的确定可实现CaO-Al2O3-V2O5渣系相图独立子体系的划分,还可为该渣系后续高温相图的绘制提供支持。-
关键词:
- 炼钢 /
- CaO-Al2O3-V2O5渣系 /
- 物相 /
- 高温相平衡试验
Abstract: CaO-Al2O3-V2O5 slag system is a basic metallurgical slag system for denitrification of liquid steel. The lack of thermodynamic information, such as the phase equilibrium relationship, limits the research and development of related slag systems. In this paper, the phase equilibrium relationship of CaO-Al2O3-V2O5 slag system was studied through high temperature phase equilibrium experiment, combined with scanning electron microscope and energy spectrum analysis. According to the experimental results, the phase equilibrium relationship of CaO-Al2O3-V2O5 slag line at 1 600 ℃ in air atmosphere was determined, and the isothermal cross section of CaO-Al2O3-V2O5 slag line within the whole component range of the system is drawn, including 7 equilibrium phase regions, i.e., Liquid+Al2O3+CaAl12O19, Liquid+CaAl12O19+CaAl4O7, Liquid+CaO, Liquid+Al2O3, Liquid+ CaAl12O19, Liquid+CaAl4O7 and single liquid phase region. The determination of equilibrium phase regions between the above phases can realize the division of independent sub-systems of the phase diagram of the CaO-Al2O3-Ce2O3 slag system, and can also provide support for the subsequent high-temperature phase diagram drawing of the slag system. -
表 1 试剂及纯度
Table 1. Reagents and their purity
% Al2O3 CaO V2O5 99.99 99.99 99.99 表 2 试验渣样设计成分
Table 2. Design compositions of experimental slag sample
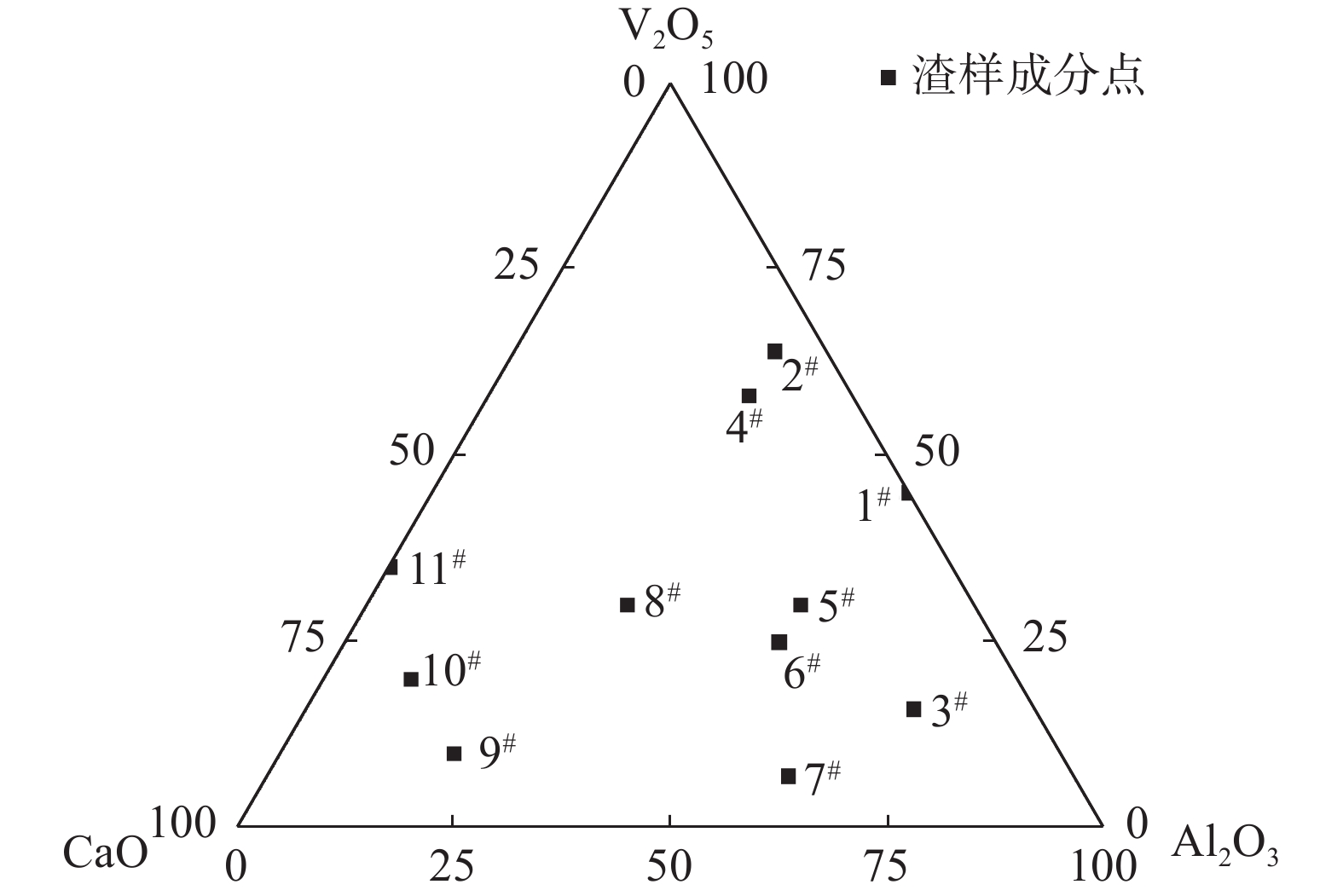
渣样编号 w(Al2O3)/% w(V2O5)/% w(CaO)/% 1# 55 45 0 2# 30 64 6 3# 70 16 14 4# 30 58 12 5# 50 30 20 6# 50 25 25 7# 60 7 33 8# 30 30 40 9# 20 10 70 10# 10 20 70 11# 0 35 65 表 3 典型渣样平衡相的能谱分析结果
Table 3. EDS compositions of typical equilibrium phases
渣样号 平衡物相 组成成分 渣样号 平衡物相 组成成分 CaO Al2O3 V2O5 CaO Al2O3 V2O5 1# 液相 7.72 92.28 7# 液相 45.19 9.93 44.89 Al2O3 97.06 2.94 CaAl4O7 19.65 81.23 2# 液相 23.88 8.91 67.21 8# 液相 34.59 53.76 11.66 Al2O3 96.03 3.98 CaAl4O7 19.34 80.66 3# 液相 33.94 8.36 57.71 9# 液相 57.75 29.26 12.99 CaAl12O19 8.05 91.95 CaO 100.00 4# 液相 23.32 10.42 66.27 10# 液相 55.77 16.71 27.53 Al2O3 93.55 6.46 CaO 100.00 5# 液相 32.01 11.51 56.49 11# 液相 54.57 45.44 CaAl12O19 7.31 92.70 CaO 100.00 Al2O3 100.00 6# 液相 42.42 7.24 50.34 CaAl4O7 20.40 79.61 CaAl12O19 9.37 90.64 -
[1] Li Xiaoming, Xi Haodong, Miao Dejun, et al. Nitrogen dissolution and control of molten steel in steelmaking process[J]. Iron and Steel, 2021,56(10):36. (李小明, 席浩栋, 缪德军, 等. 炼钢流程钢中氮的溶解及控制技术[J]. 钢铁, 2021,56(10):36.Li Xiaoming, Xi Haodong, Miu Dejun, et al. Nitrogen dissolution and control of molten steel in steelmaking process[J]. Iron and steel, 2021, 56(10): 36 [2] Wang Min, Bao Yanping, Cui Heng, et al. Nitrogen behavior in CaO-SiO2-Al2O3-MgO refining slags[J]. Journal of University of Science and Technology, 2010,32(2):175-178. (王敏, 包燕平, 崔衡, 等. CaO-SiO2-Al2O3-MgO精炼渣氮行为[J]. 北京科技大学学报, 2010,32(2):175-178.Wang Min, Bao Yanping, Cui Heng, et al. Nitrogen behavior in CaO-SiO2-Al2O3-MgO refining slags[J]. Journal of University of Science and Technology, 2010, 32(2): 175-178 [3] Chang Heming, Zhang Lijuan. Kinetic model of denitrogenization of molten steel under vacuum with bubbling[J]. Journal of Iron and Steel Research, 1997,9(4):5-8. (唱鹤鸣, 张丽娟. 熔渣下钢液真空吹气脱氮动力学模型[J]. 钢铁研究学报, 1997,9(4):5-8.Chang Heming, Zhang Lijuan. Kinetic model of denitrogenization of molten steel under vacuum with bubbling[J]. Journal of iron and steel research, 1997, 9(04): 5-8 [4] 石霖. 合金热力学[M]. 北京: 机械工业出版社, 1992.Shi Lin. Alloy thermodynamics[M]. Beijing: China Machine Press, 1992. [5] Yang Y, Mao H, Selleby M. An assessment of the Ca-V-O system[J]. Calphad, 2017,56:29-40. doi: 10.1016/j.calphad.2016.11.005 [6] Morozov A N. System CaO-V2O5[J]. Metallurg (Leningrad), 1938,13:21-28. [7] Li Xugang, Xie Wei, Wang Nai, et al. Thermodynamic optimization of Al2O3-V2O5 system[J]. Nonferrous Metals Science and Engineering, 2019,10(6):13-18. (李徐刚, 谢伟, 王耐, 等. Al2O3-V2O5体系的热力学优化[J]. 有色金属科学与工程, 2019,10(6):13-18.Li Xugang, Xie Wei, Wang Nai, et al. Thermodynamic optimization of Al2O3-V2O5 system[J]. Nonferrous Metals Science and Engineering, 2019, 10(6): 13-18 [8] Dabrowska G, Tabero P M K. Phase relations in the Al2O3-V2O5-MoO3 system in the solid state: The crystal structure of AlVO4[J]. Journal of Phase Equilibria and Diffusion, 2009,30(3):220-229. doi: 10.1007/s11669-009-9503-4 [9] Liu Chengjun, Qiu Jiyu. Phase equilibrium relations in the specific region of CaO-SiO2-La2O3 system[J]. Journal of the European Ceramic Society, 2018,38(4):2090-2097. doi: 10.1016/j.jeurceramsoc.2017.12.011 [10] Liu Chengjun, Qiu Jiyu, Liu Zhengyue, et al. Adjacent relations of primary phase fields and invariant reactions of the system CaO-SiO2-Nb2O5-La2O3[J]. Journal of The American Ceramic Society, 2021,104(5):2398-2409. doi: 10.1111/jace.17605 [11] Liu Chengjun, Qiu Jiyu, Liu Zhengyue, et al. Phase equilibria in the system CaO-SiO2-Nb2O5-La2O3 at 1473 K with pO2=10-15.47 atm[J]. Ceramics International, 2020,46(4):7711-7718. [12] Qiu Jiyu, Liu Chengjun, Liu Zhengyue, et al. Phase equilibria in the CaO-La2O3-Nb2O5 system at 1823, 1773, and 1673 K[J]. Journal of Asian Ceramic Societies, 2020,8(3):764-776. doi: 10.1080/21870764.2020.1789288 -




 下载:
下载: