Research on activator enhanced acid leaching process of titanium concentrate
-
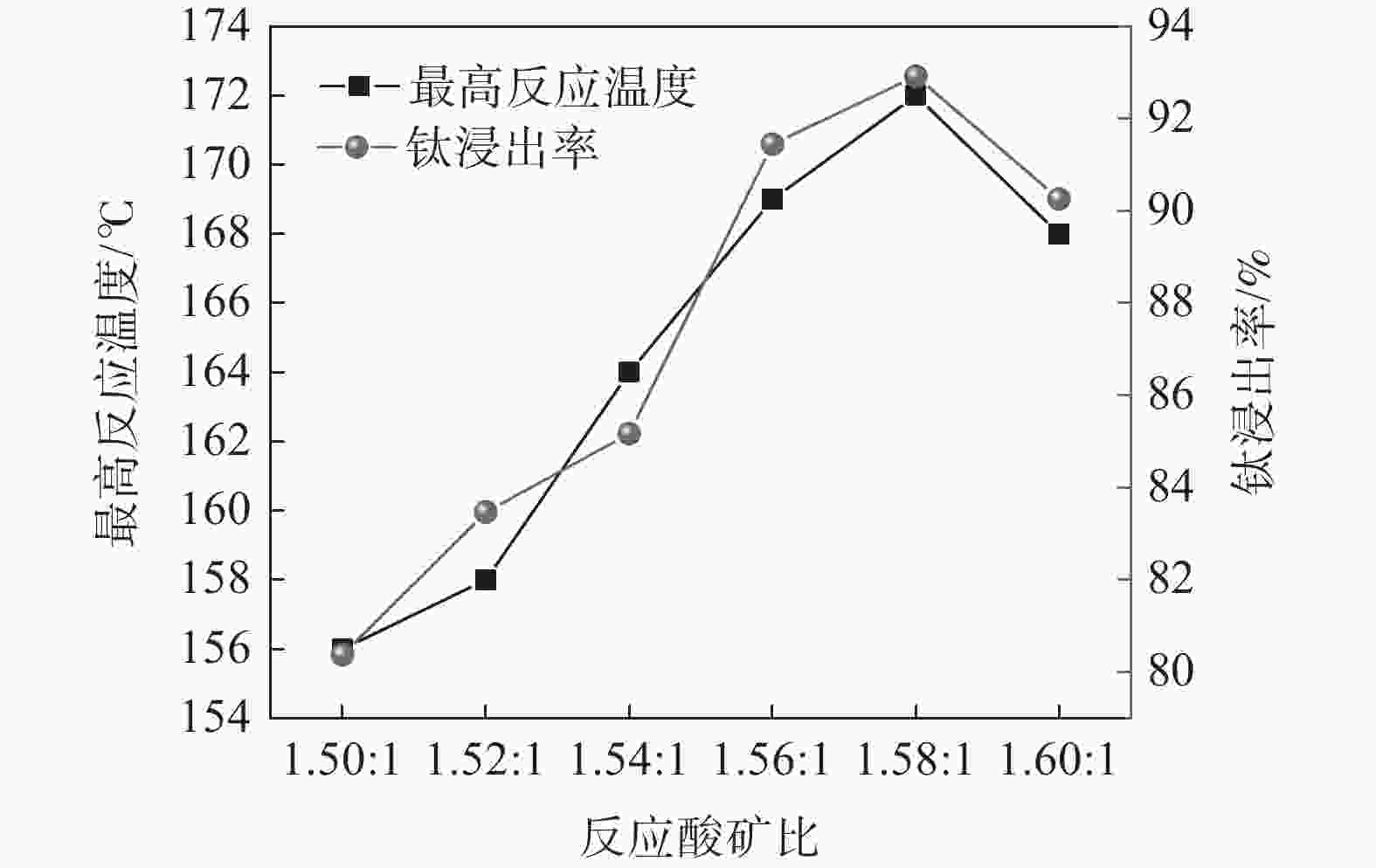
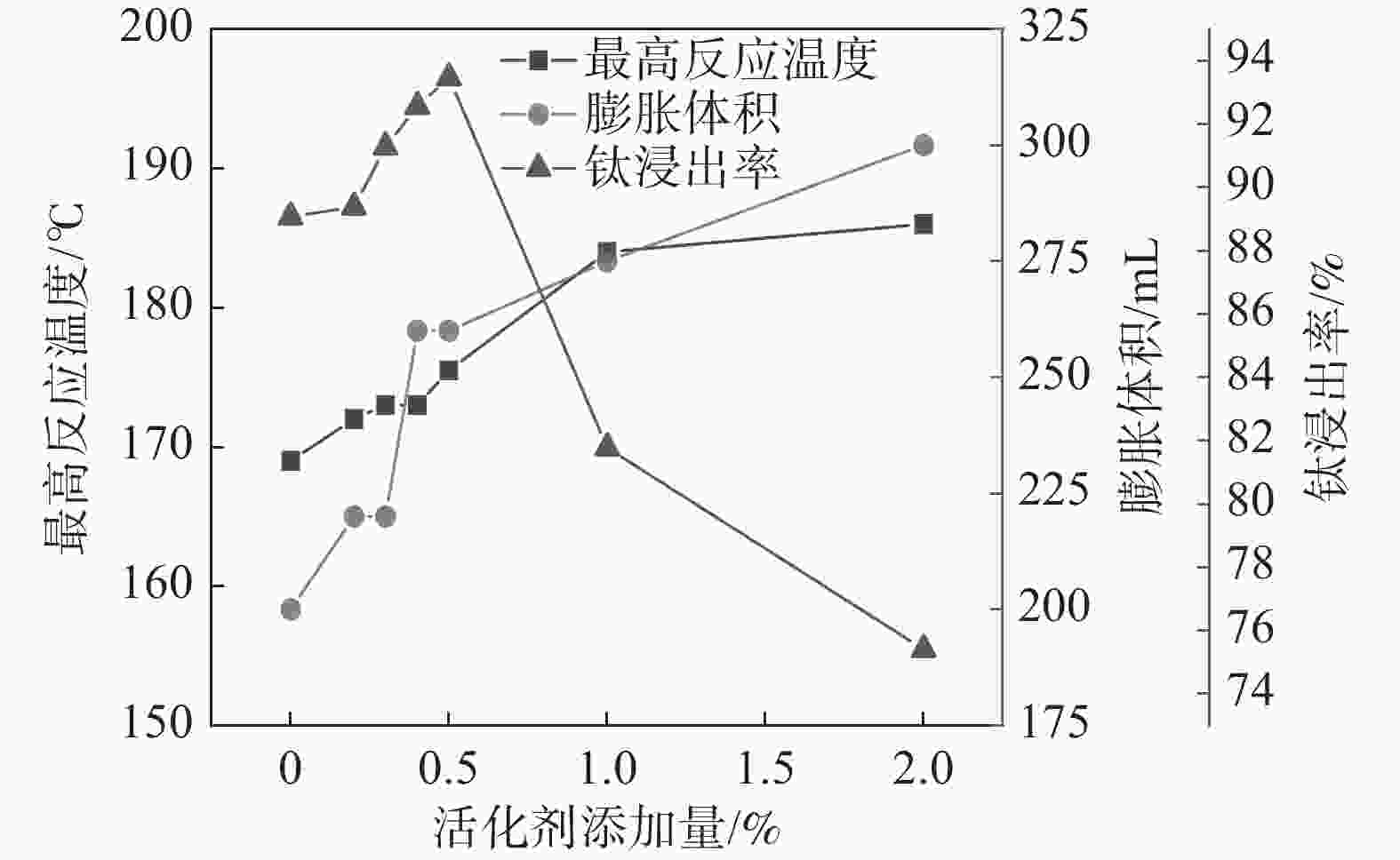
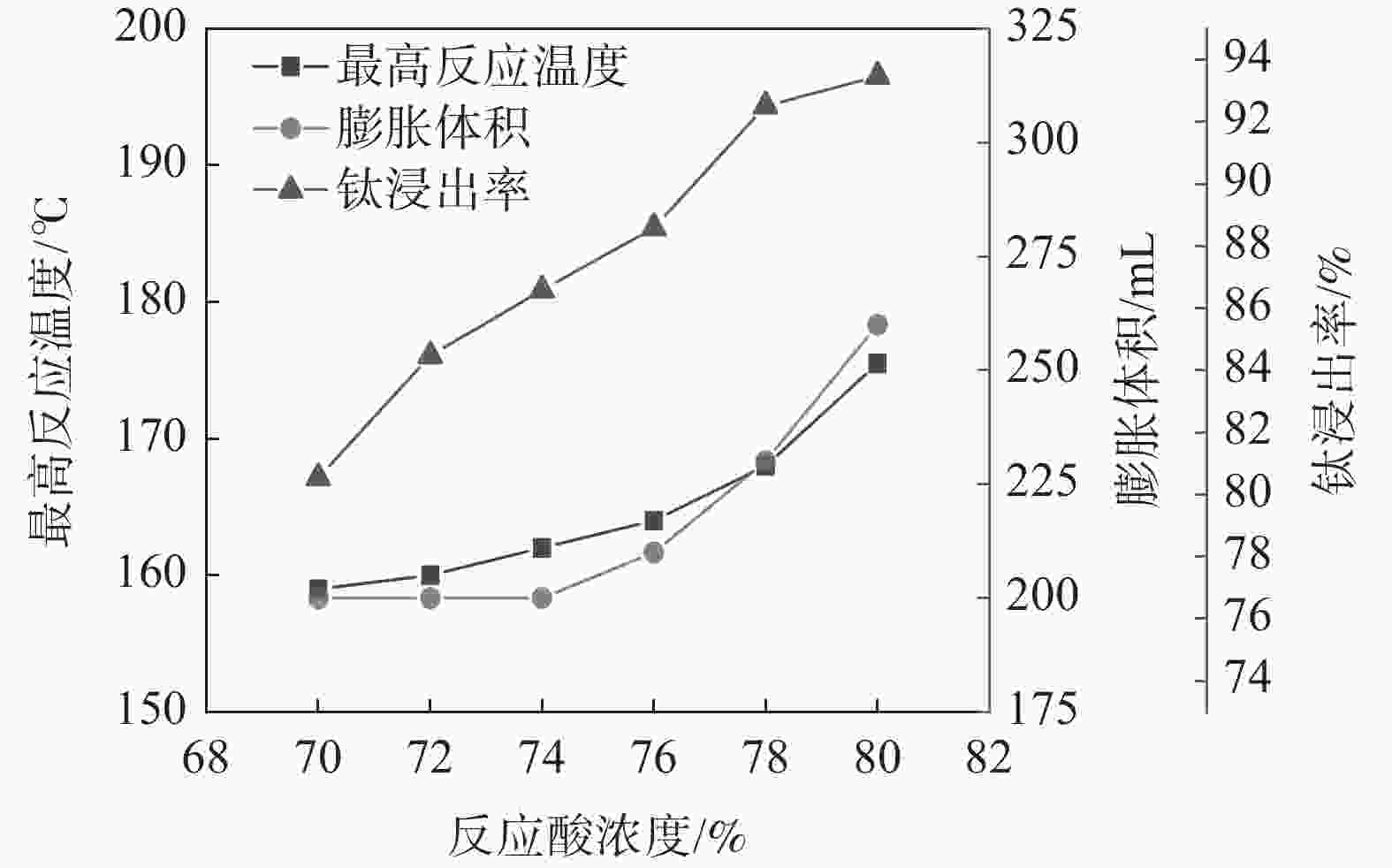
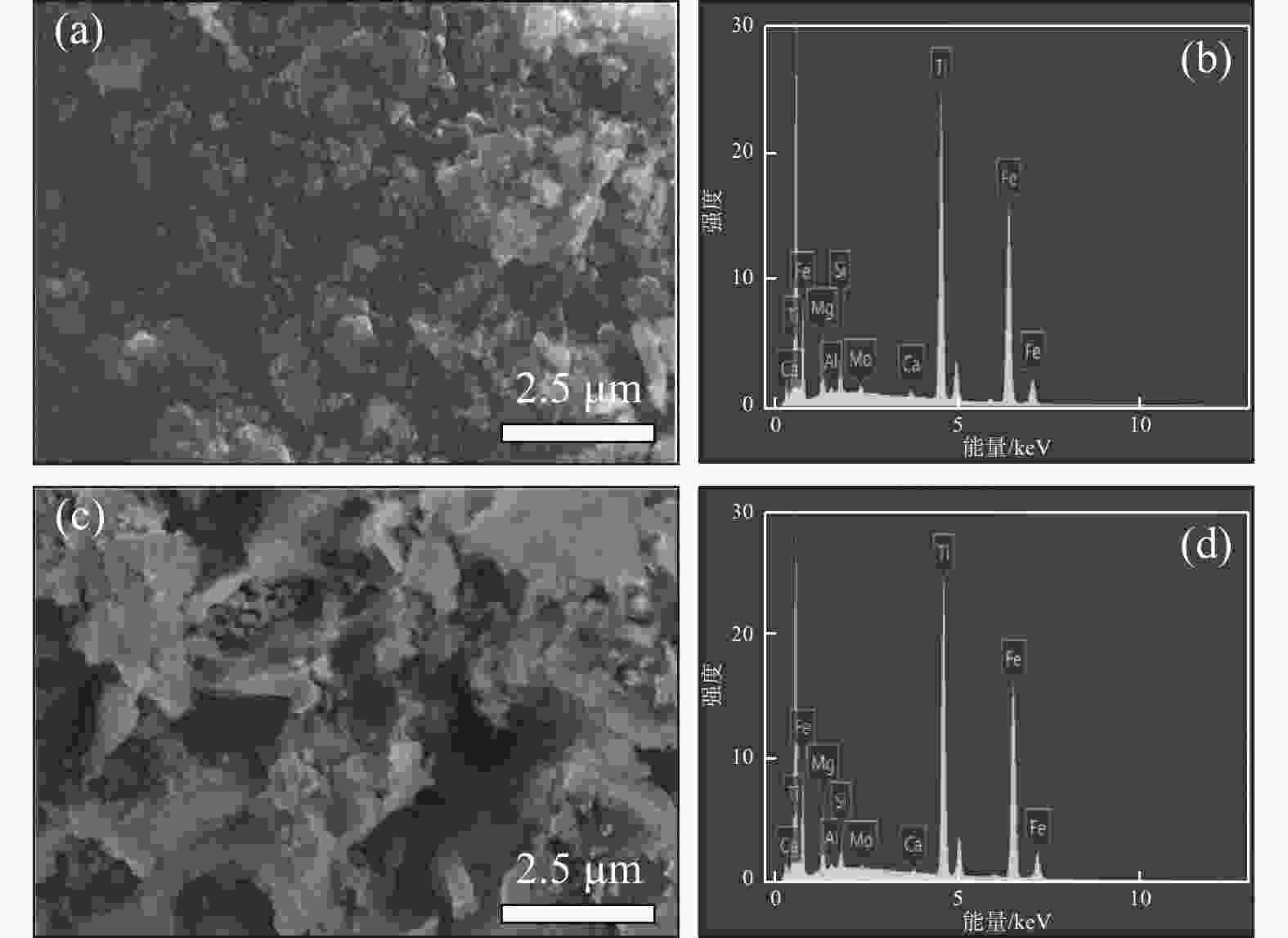
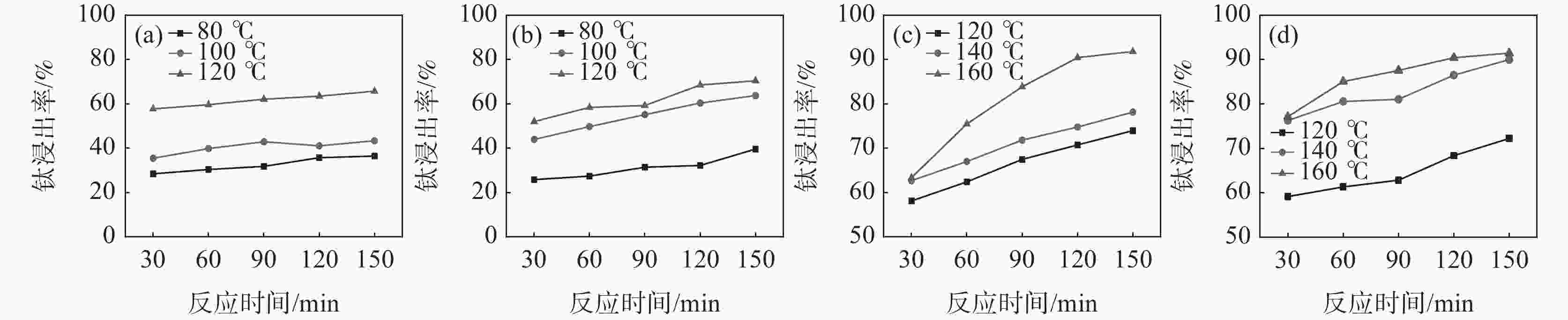
摘要: 针对硫酸法钛白生产中反应酸浓度较高导致不能实现硫酸平衡的问题,通过引入活化剂强化酸解反应过程,降低反应酸浓度。研究了活化剂对钛精矿低浓度酸浸的强化作用,考察了反应酸浓度和活化剂加量对反应温度和钛浸出率的影响。结果表明:活化剂的加入,可以增加反应放热量,提高反应温度,并改善固相物的疏松度,浸出活性更高;活化剂加量为0.5%、酸矿比为1.56∶1,反应酸浓度为80%的条件下,钛精矿的钛浸出率达到93.47%;酸浸残渣主要成分为TiFeO3、Ca(Fe, Mg)Si2O6和SiO2,表明原料中的钛大部分被转移到液相中。Abstract: In response to the problem of high reaction acid concentration in the production of titanium dioxide by sulfuric acid method, which leads to the inability to achieve sulfuric acid equilibrium, an activator is introduced to enhance the acid hydrolysis reaction process and reduce the reaction acid concentration. The strengthening effect of the activator on the low concentration acid leaching of titanium concentrate was studied, and the effects of reaction acid concentration and activator dosage on reaction temperature and acid leaching rate were investigated. The results show that the addition of activating agent can increase the heat release of the reaction, increase the reaction temperature, and improve the porosity of the solid phase, resulting in higher leaching activity. Under the conditions of 0.5% activator dosage, 1.56:1 acid to ore ratio, and 80% reaction acid concentration, the acid hydrolysis rate of titanium concentrate reaches 93.47%. The main components of acid leaching residue are TiFeO3, Ca(Fe, Mg)Si2O6 and SiO2, indicating that most of the titanium in the raw material can be transferred to the liquid phase.
-
Key words:
- titanium concentrate /
- acid leaching /
- activator /
- solid phase
-
表 1 不同废酸浓度下酸平衡时的反应酸浓度
Table 1. Reaction acid concentrations at acid equilibrium under different wasts acid concentration
酸矿比 不同废酸浓度(%)下反应酸浓度/% 20 30 40 45 50 55 60 1.52 47.32 60.40 70.09 74.0 77.56 80.68 83.48 1.53 47.48 60.56 70.22 74.17 77.66 80.77 83.56 1.54 47.64 60.71 70.35 74.29 77.77 80.86 83.64 1.55 47.80 60.86 70.48 74.40 77.87 80.96 83.72 1.56 47.96 61.00 70.61 74.52 77.97 81.05 83.80 表 2 不同反应酸浓度下酸浸反应参数
Table 2. Acid leaching reaction parameters under different reaction acid concentrations
反应酸浓度/% 沸点/ ℃ 最高反应温度/ ℃ 50 124 119 60 141.8 128 70 162.2 138 80 200 169 84 221.3 179 表 3 酸浸残渣成分分析
Table 3. Compositions of acid hydrolysis residue
% 成分 SiO2 Fe2O3 MgO S TiO2 CaO MnO Cr2O3 未加活化剂 1.75 20.77 0.72 4.65 31.61 1.09 0.32 0.67 活化剂加量0.5% 3.69 12.31 0.44 6.41 21.70 2.85 0.10 0.07 -
[1] Bi Sheng. Status, future and development of China’s titanium dioxide industry in 2023[J]. Iron Steel Vanadium Titanium, 2024,45(1):1-3. (毕胜. 2023年中国钛白粉行业的现状、未来及发展[J]. 钢铁钒钛, 2024,45(1):1-3. doi: 10.7513/j.issn.1004-7638.2024.01.001Bi Sheng. Status, future and development of China’s titanium dioxide industry in 2023[J]. Iron Steel Vanadium Titanium, 2024, 45(1): 1-3. doi: 10.7513/j.issn.1004-7638.2024.01.001 [2] Wang Haibo, Sun Ke. Study on concentration process of titanium white waste acid by sulfuric acid method[J]. Iron Steel Vanadium Titanium, 2023,44(5):116-121. (王海波, 孙科. 硫酸法钛白废酸浓缩工艺研究[J]. 钢铁钒钛, 2023,44(5):116-121. doi: 10.7513/j.issn.1004-7638.2023.05.018Wang Haibo, Sun Ke. Study on concentration process of titanium white waste acid by sulfuric acid method[J]. Iron Steel Vanadium Titanium, 2023, 44(5): 116-121. doi: 10.7513/j.issn.1004-7638.2023.05.018 [3] Xu Bowen, Zhang Tao, Li Lv, et al. Analysis of the composition and formation mechanism of fouling in the concentration process of titanium white waste acid[J]. Industrial & Engineering Chemistry Research, 2023,62(23):9325-9334. [4] Wang Haibo, Wang Kui, Sun Ke, et al. Cause analysis of blockage in heat exchanger of titanium white waste acid concentration by sulfuric acid method[J]. Iron Steel Vanadium Titanium, 2021,42(5):115-119. (王海波, 王奎, 孙科, 等. 硫酸法钛白废酸浓缩换热器堵塞成因分析[J]. 钢铁钒钛, 2021,42(5):115-119. doi: 10.7513/j.issn.1004-7638.2021.05.018Wang Haibo, Wang Kui, Sun Ke, et al. Cause analysis of blockage in heat exchanger of titanium white waste acid concentration by sulfuric acid method[J]. Iron Steel Vanadium Titanium, 2021, 42(5): 115-119. doi: 10.7513/j.issn.1004-7638.2021.05.018 [5] Wang Minghua, Du Xinghong, Sui Zhitong. Kinetics of acidolysis of rich titanium concentrate by H2SO4[J]. The Chinese Journal of Nonferrous Metals, 2001,11(1):131-134. (王明华, 都兴红, 隋智通. H2SO4分解富钛精矿的反应动力学[J]. 中国有色金属学报, 2001,11(1):131-134. doi: 10.3321/j.issn:1004-0609.2001.01.029Wang Minghua, Du Xinghong, Sui Zhitong. Kinetics of acidolysis of rich titanium concentrate by H2SO4[J]. The Chinese Journal of Nonferrous Metals, 2001, 11(1): 131-134. doi: 10.3321/j.issn:1004-0609.2001.01.029 [6] Maciej Jabłoński, Sandra Tylutka. The influence of initial concentration of sulfuric acid on the degree of leaching of the main elements of ilmenite raw materials[J]. J. Therm Anal Calorim, 2015, 124:355-361. [7] Du Jun, Li Chun, Yuan Shaojun, et al. A coupling process of mechanical activation and acid digestion of ilmenite[J]. Chemical Reaction Engineering and Technology, 2014,30(4):321-328. (杜均, 李春, 袁绍军, 等. 钛铁矿机械活化-稀酸酸解反应耦合[J]. 化学反应工程与工艺, 2014,30(4):321-328.Du Jun, Li Chun, Yuan Shaojun, et al. A coupling process of mechanical activation and acid digestion of ilmenite[J]. Chemical Reaction Engineering and Technology, 2014, 30(4): 321-328. [8] Wang Xiaomei, Li Chun,Yue Hairong, et al. Effects of mechanical activation on the digestion of ilmenite in dilute H2SO4[J]. Chinese Journal of Chemical Engineering, 2019,27:575-586. doi: 10.1016/j.cjche.2018.06.020 [9] Wu Jianchun, Lu Ruifang, Shi Ruicheng. Study on the acidolysis properties of titanium ore recovered from acidolysis residue[J]. Iron Steel Vanadium Titanium, 2021,42(3):37-43. (吴健春, 路瑞芳, 石瑞成. 酸解残渣回收矿的酸解性能研究[J]. 钢铁钒钛, 2021,42(3):37-43. doi: 10.7513/j.issn.1004-7638.2021.03.006Wu Jianchun, Lu Ruifang, Shi Ruicheng. Study on the acidolysis properties of titanium ore recovered from acidolysis residue[J]. Iron Steel Vanadium Titanium, 2021, 42(3): 37-43. doi: 10.7513/j.issn.1004-7638.2021.03.006 [10] Nayl A A, Awwad N S, AlyH F. Kinetics of acid leaching of ilmenite decomposed by KOH Part 2. Leaching by H2SO4 and C2H2O4[J]. Journal of Hazardous Materials, 2009,168:793-799. doi: 10.1016/j.jhazmat.2009.02.076 [11] Liu Qingsheng, Tu Tao, Guo Hao, et al. Complexation extraction of scheelite and transformation behavior of tungsten-containing phase using H2SO4 solution with H2C2O4 as complexing agent[J]. Trans. Nonferrous Met. Soc. China, 2021, 31: 3150-3161. [12] Wu Aixiang, Ai Chunming, Wang Yiming, et al. Influence of surfactant on permeability of heap leaching of copper ore[J]. Journal of Central South University (Science and Technology), 2014,45(3):895-901. (吴爱祥, 艾纯明, 王贻明, 等. 表面活性剂对铜矿石堆浸渗透性的影响[J]. 中南大学学报(自然科学版), 2014,45(3):895-901.Wu Aixiang, Ai Chunming, Wang Yiming, et al. Influence of surfactant on permeability of heap leaching of copper ore[J]. Journal of Central South University (Science and Technology), 2014, 45(3): 895-901. [13] Tian Hong, Ma Mengyu, Ye Hengpeng, et al. Leaching of manganese from low-grade manganese ore using sulfuric acid with synthesized atmospheric surfactant[J]. Hydrometallurgy of China, 2021, 40(4): 272-277. (田鸿, 马梦雨, 叶恒朋, 等. 用两性表面活性剂辅助浸出低品位锰矿石中的锰[J]. 湿法冶金, 2021, 40(4): 272-277.Tian Hong, Ma Mengyu, Ye Hengpeng, et al. Leaching of manganese from low-grade manganese ore using sulfuric acid with synthesized atmospheric surfactant[J]. Hydrometallurgy of China, 2021, 40(4): 272-277. [14] YB/T 159.1-2015. Titanium concentrate (rock minerals)- Determination of titanium dioxide content- The ferric ammonium sulfate titrimetric method[S]. Beijin: Metallurgical industry press, 2015. (YB/T 159.1-2015 .钛精矿(岩矿)二氧化钛含量的测定 硫酸铁铵滴定法[S]. 北京: 冶金工业出版社, 2015.YB/T 159.1-2015. Titanium concentrate (rock minerals)- Determination of titanium dioxide content- The ferric ammonium sulfate titrimetric method[S]. Beijin: Metallurgical industry press, 2015. [15] Ma Weiping. Research on acidolysis of Panxin titanium concentrate with sulfuric acid[J]. Inorganic Chemicals Industry, 2013,45(5):24-26. (马维平. 硫酸酸解攀西钛精矿技术研究[J]. 无机盐工业, 2013,45(5):24-26. doi: 10.3969/j.issn.1006-4990.2013.05.008Ma Weiping. Research on acidolysis of Panxin titanium concentrate with sulfuric acid[J]. Inorganic Chemicals Industry, 2013, 45(5): 24-26. doi: 10.3969/j.issn.1006-4990.2013.05.008 [16] Maciej Jabłoński, Sandra Tylutka. The influence of initial concentration of sulfuric acid on the degree of leaching of the main elements of ilmenite raw materials[J]. J Therm Anal Calorim, 2016,124:355-361. doi: 10.1007/s10973-015-5114-y [17] Wang Haibo, Wu Xiaoping, Gao Jian, et al. Kinetics of sulfuric acid leaching of ilmenite[J]. Iron Steel Vanadium Titanium, 2020,41(6):6-10. (王海波, 吴小平, 高健, 等. 硫酸浸取钛铁矿动力学研究[J]. 钢铁钒钛, 2020,41(6):6-10. doi: 10.7513/j.issn.1004-7638.2020.06.002Wang Haibo, Wu Xiaoping, Gao Jian, et al. Kinetics of sulfuric acid leaching of ilmenite[J]. Iron Steel Vanadium Titanium, 2020, 41(6): 6-10. doi: 10.7513/j.issn.1004-7638.2020.06.002 -




 下载:
下载: