Preparation of titanium by hydrogenation and analysis of its energy
-
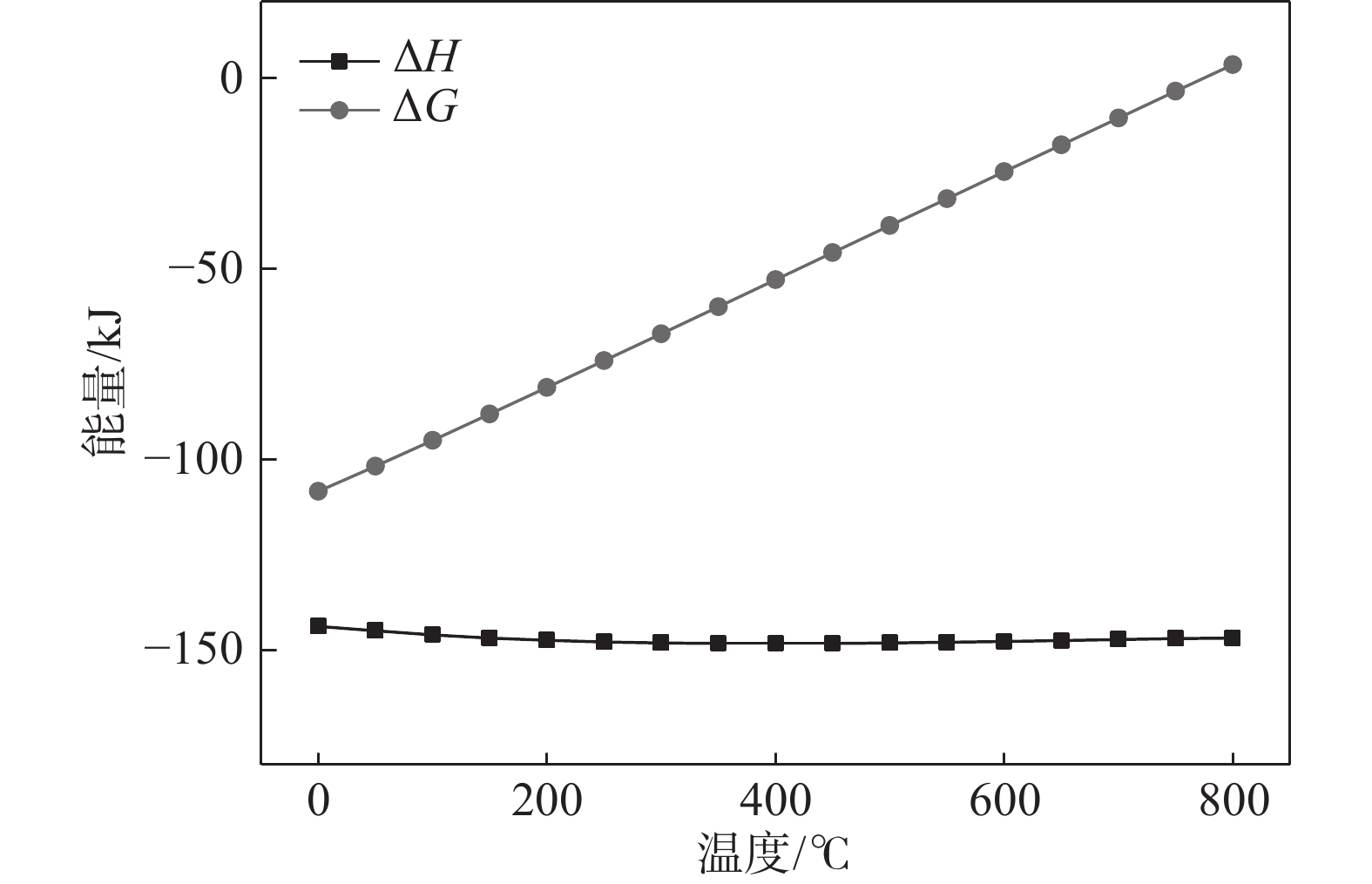
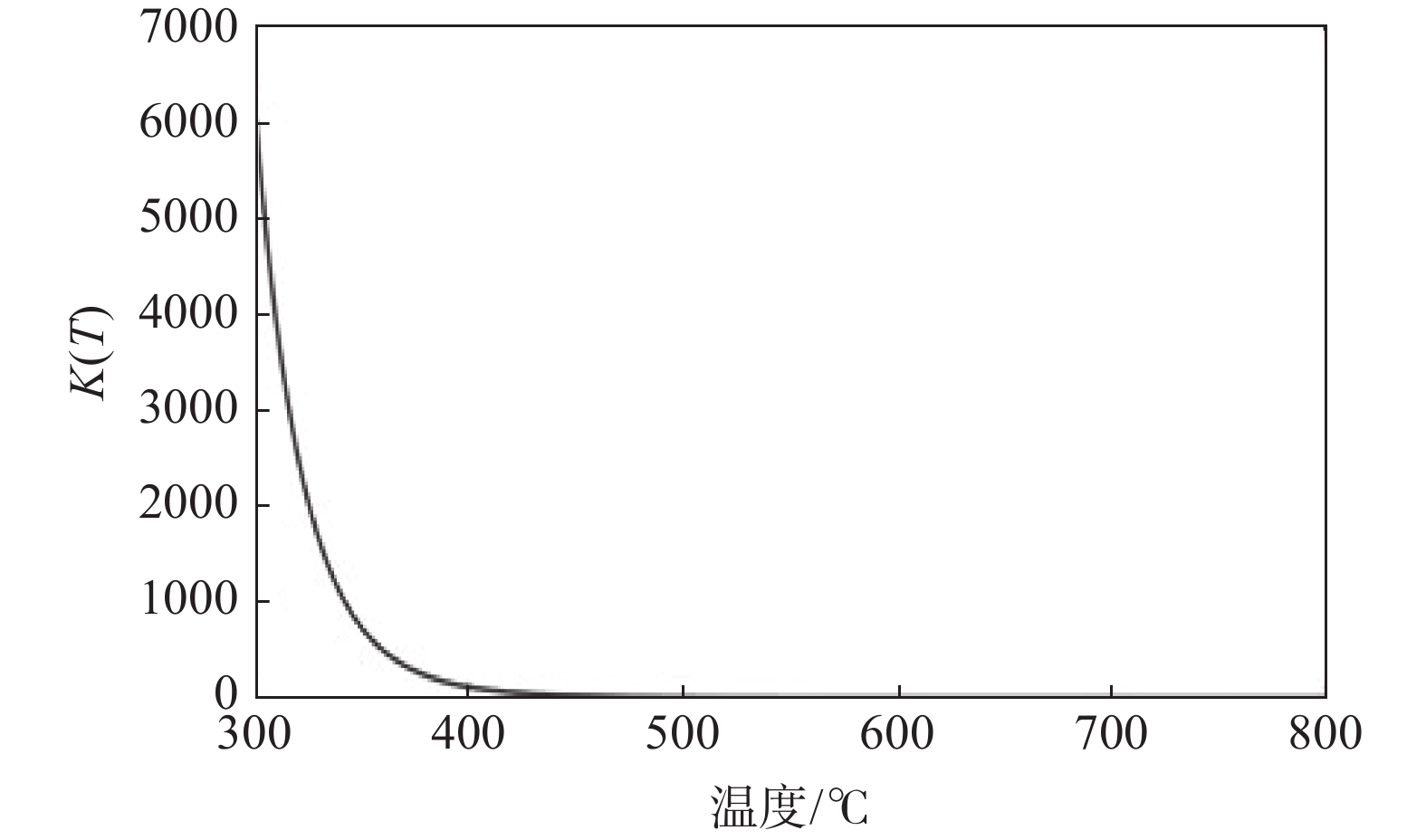
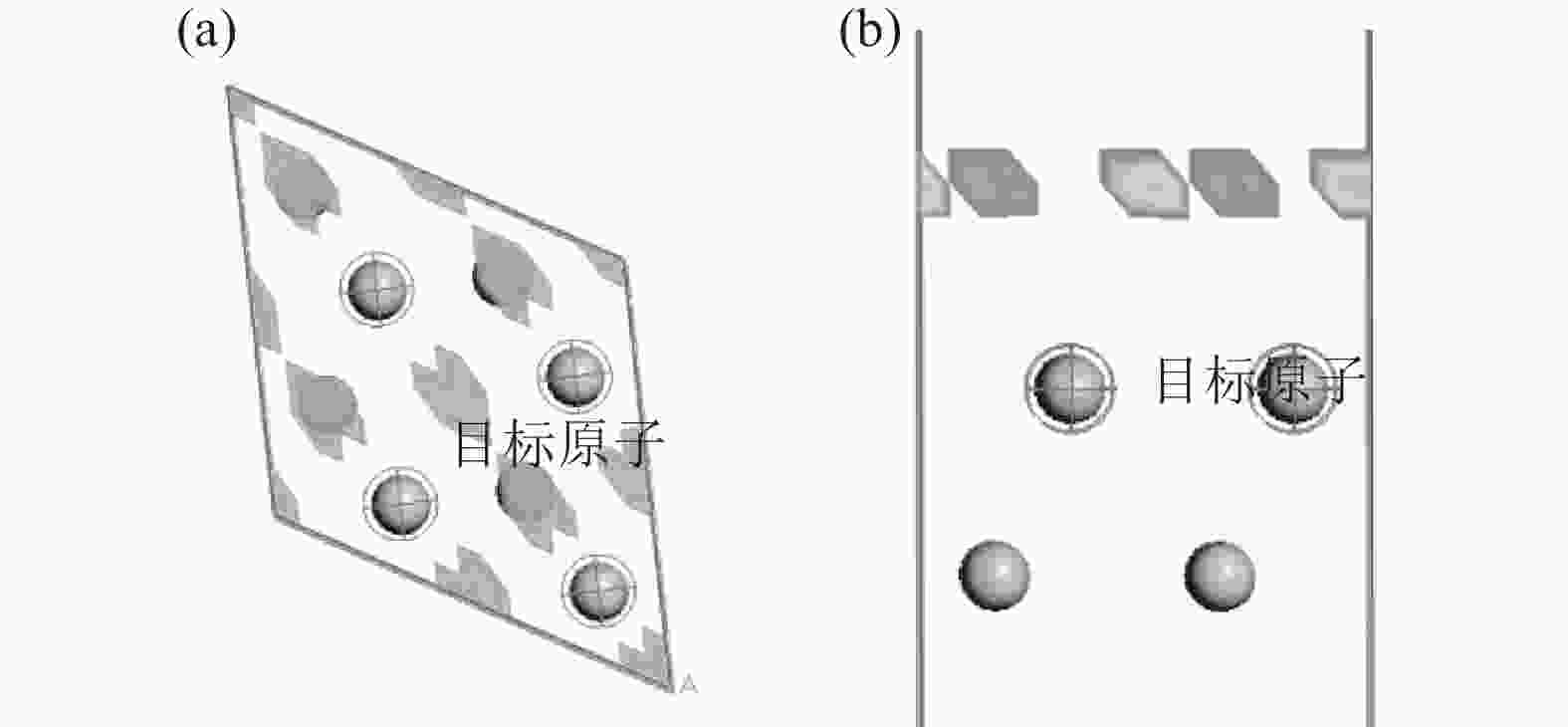
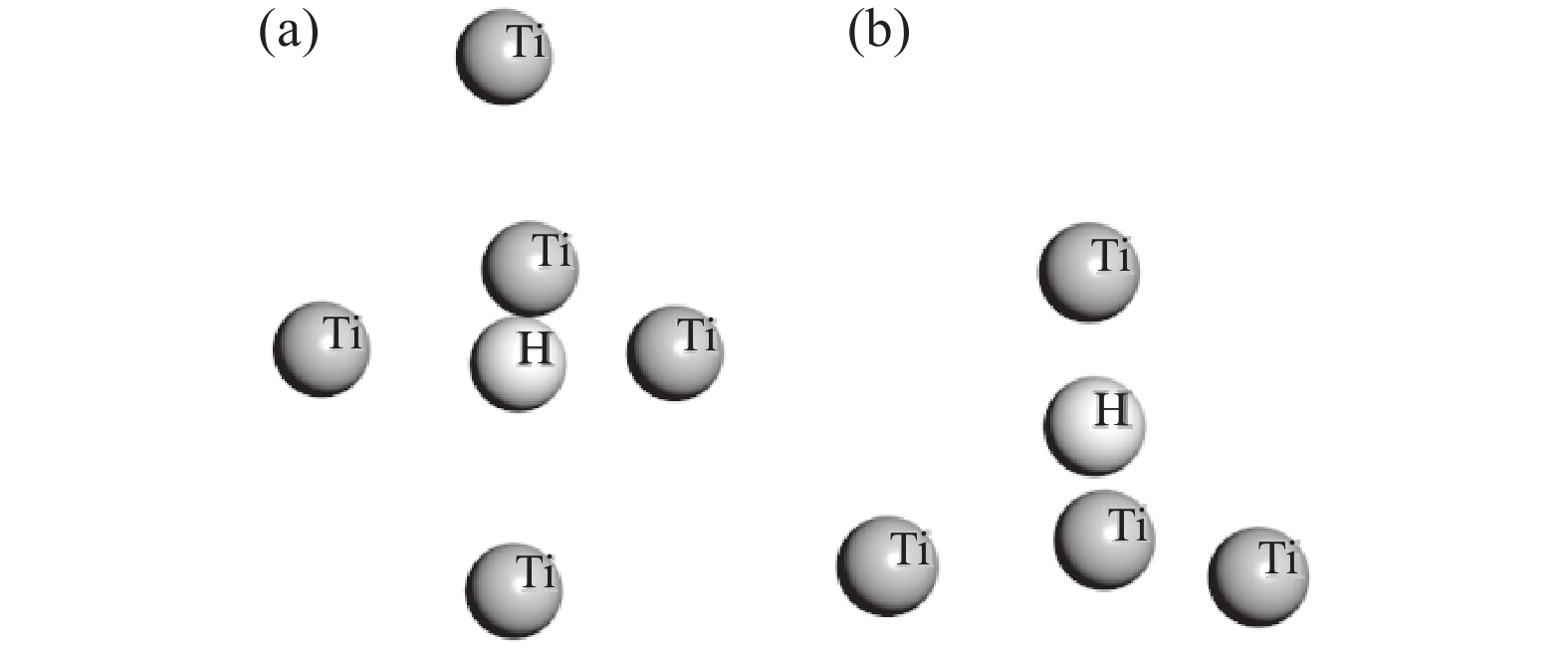
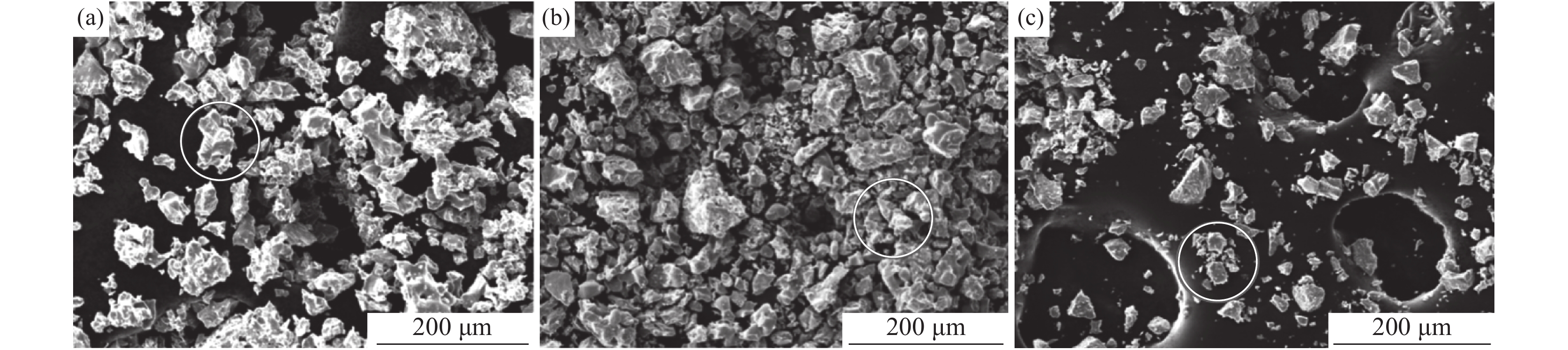
摘要: 以海绵钛为原料,在不同温度、不同时间下制备出氢化钛。根据重量变化分析其反应原理,用X射线衍射仪(XRD)进行结构分析,扫描电镜(SEM)进行形貌分析。结果显示:随反应温度升高,氢化钛质量增加率增大,脆性也变大。当温度高于500 ℃之后,质量增长率变化不大。热力学计算结果发现温度从300 ℃增加到700 ℃时,平衡常数显著下降,表明反应温度过高不利于反应进行。氢化过程的吸附能量计算表明其较优吸附位位于其中心点位。固溶能量分析表明氢原子更倾向于占据八面体间隙。Abstract: Titanium hydride was prepared from sponge titanium at different temperatures and time. Perform structural analysis using X-ray diffraction (XRD) and morphology analysis using scanning electron microscopy (SEM). The results show that with the increase of reaction temperature, the mass growth rate of titanium hydride increases, and the brittleness also increases. When the temperature exceeds 500 ℃, there is little change in the mass growth rate. The thermodynamic calculation results show that when the temperature increases from 300 ℃ to 700 ℃, the equilibrium constant significantly decreases, indicating that a high reaction temperature is not conducive to the reaction. The calculation of adsorption energy in the hydrogenation process shows that the optimal adsorption site is located at its central point. Solid solution energy analysis shows that hydrogen atoms tend to occupy octahedral gaps more.
-
Key words:
- titanium hydride /
- sponge titanium /
- reaction temperature /
- prepartion /
- characterization
-
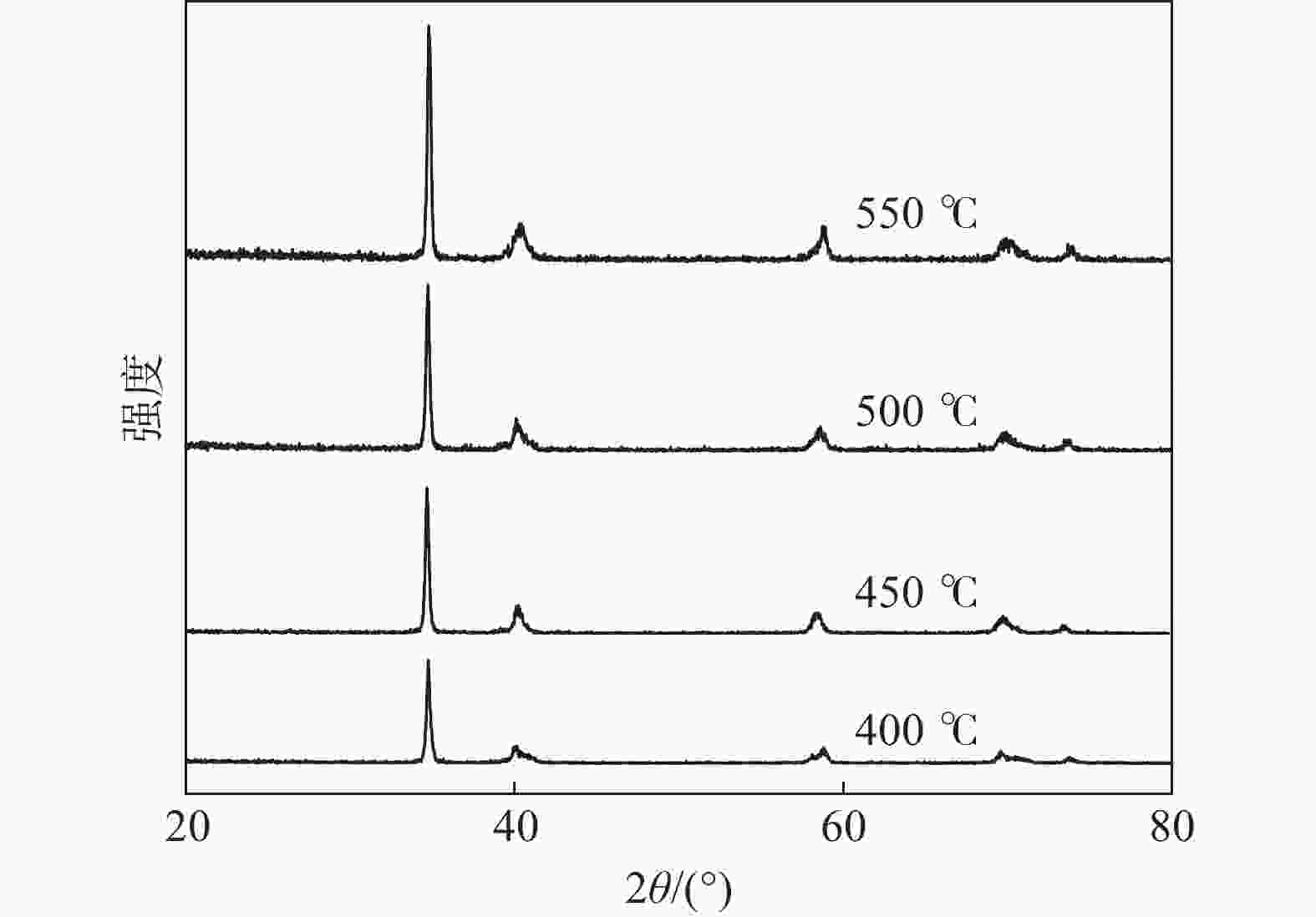
表 1 不同温度下质量增长率及脆性
Table 1. Quality growth rate and brittleness at different temperatures
反应温度/℃ 钛质量/g 氢化钛质量/g 质量增加率/% 脆性 400 1.286 1.335 3.8 小 450 2.745 2.858 4.1 小 500 2.225 2.318 4.2 一般 550 2.811 2.929 4.2 大 表 2 氢原子占据α-Ti-H 体系八面体和四面体间隙的溶解热计算结果
Table 2. Calculation results of dissolution heat of hydrogen atoms occupying between octahedral and tetrahedral gaps in α-Ti-H system
晶体型 位置 Et/eV ΔH(eV·atom-1) α-Ti(2Ti) − 3212.24528 0 16Ti-H 八面体间隙 − 25714.25413 − 0.4082 8Ti-H 八面体间隙 − 12865.36544 − 0.5007 4Ti-H 八面体间隙 − 6425.06412 − 0.57356 2Ti-H 八面体间隙 − 3212.90524 − 0.65996 16Ti-H 四面体间隙 − 3206.24854 − 0.31926 8Ti-H 四面体间隙 − 12841.24458 − 0.36677 4Ti-H 四面体间隙 − 6428.81731 − 0.43658 2Ti-H 四面体间隙 − 3222.60511 − 0.47292 表 3 氢原子位于八面体间隙的α-Ti-H 体系的体积变化
Table 3. Volume change of α Ti-H system of hydrogen atoms located in octahedral gaps
% α-Ti(2Ti) 16Ti-H 8Ti-H 4Ti-H 2Ti-H 0 0.17 0.87 1.17 3.84 -
[1] Feng Yingfang, Zhang Zhen. Production and application of titanium powder[J]. Titanium Industry Progress, 2000(6):6-9. (冯颖芳, 张震. 钛粉的生产及应用[J]. 钛工业进展, 2000(6):6-9. doi: 10.3969/j.issn.1009-9964.2000.06.002Feng Yingfang, Zhang Zhen. Production and application of titanium powder[J]. Titanium Industry Progress, 2000(6): 6-9. doi: 10.3969/j.issn.1009-9964.2000.06.002 [2] Hong Yan, Qu Tao, Shen Huasen, et al. Titanium production through hydrogenation and dehydrogenation process[J]. Chinese Journal of Rare Metals, 2007(3):311-315. (洪艳, 曲涛, 沈化森, 等. 氢化脱氢法制备钛粉工艺研究[J]. 稀有金属, 2007(3):311-315. doi: 10.3969/j.issn.0258-7076.2007.03.007Hong Yan, Qu Tao, Shen Huasen, et al. Titanium production through hydrogenation and dehydrogenation process[J]. Chinese Journal of Rare Metals, 2007(3): 311-315. doi: 10.3969/j.issn.0258-7076.2007.03.007 [3] Huang Guangming, Lei Ting, Fang Shuming, et al. Research progress of preparation powders by titanium hydrogenation[J]. Titanium Industry Progress, 2010(6):6-9. (黄光明, 雷霆, 方树铭, 等. 氢化脱氢制备钛粉的研究进展[J]. 钛工业进展, 2010(6):6-9. doi: 10.3969/j.issn.1009-9964.2010.05.002Huang Guangming, Lei Ting, Fang Shuming, et al. Research progress of preparation powders by titanium hydrogenation[J]. Titanium Industry Progress, 2010(6): 6-9. doi: 10.3969/j.issn.1009-9964.2010.05.002 [4] Liu Jie, Shang Qingliang, Zhang Wei, et al. Research progress in preparation of titanium and titanium alloy materials by titanium hydride powder[J]. Materials Review, 2013,13:99-102. (刘捷, 尚青亮, 张炜, 等. 氢化钛粉制备钛及钛合金材料研究进展[J]. 材料导报, 2013,13:99-102. doi: 10.3969/j.issn.1005-023X.2013.01.019Liu Jie, Shang Qingliang, Zhang Wei, et al. Research progress in preparation of titanium and titanium alloy materials by titanium hydride powder[J]. Materials Review, 2013, 13: 99-102. doi: 10.3969/j.issn.1005-023X.2013.01.019 [5] Chen Song, Xiao Sufen, Wang Chunming, et al. Hydrogen dehydrogenation kinetics of titanium sponge from Panzhihua[J]. Functional Materials, 2014,45(11):11123-11125, 11131. (陈松, 肖素芬, 王春明, 等. 攀枝花产海绵钛氢化脱氢动力学[J]. 功能材料, 2014,45(11):11123-11125, 11131. doi: 10.3969/j.issn.1001-9731.2014.11.026Chen Song, Xiao Sufen, Wang Chunming, et al. Hydrogen dehydrogenation kinetics of titanium sponge from Panzhihua[J]. Functional Materials, 2014, 45(11): 11123-11125, 11131. doi: 10.3969/j.issn.1001-9731.2014.11.026 [6] Han Xiuli, Wang Qing, Sun Dongli, et al. First-principle calculation of crystal structure and energies of Ti-H system[J]. The Chinese Journal of Nonferrous Metals, 2008(3):523-528. (韩秀丽, 王清, 孙东立, 等. 钛-氢体系晶体结构和能量的第一原理计算[J]. 中国有色金属学报, 2008(3):523-528. doi: 10.3321/j.issn:1004-0609.2008.03.024Han Xiuli, Wang Qing, Sun Dongli, et al. First-principle calculation of crystal structure and energies of Ti-H system[J]. The Chinese Journal of Nonferrous Metals, 2008(3): 523-528. doi: 10.3321/j.issn:1004-0609.2008.03.024 [7] Lu Jinlian, Cao Juexian. A first-principles study of capacity and mechanism of a single titanium atom storing hydrogen[J]. Acta Physica Sinica, 2012,45(14):497-502. (卢金炼, 曹觉先. 单个钛原子储氢能力和储氢机制的第一性原理研究[J]. 物理学报, 2012,45(14):497-502. doi: 10.7498/aps.61.148801Lu Jinlian, Cao Juexian. A first-principles study of capacity and mechanism of a single titanium atom storing hydrogen[J]. Acta Physica Sinica, 2012, 45(14): 497-502. doi: 10.7498/aps.61.148801 [8] Zhang Jian, Zhou Dianwu, Liu Jinshui. Study on hydrogen atom adsorption and diffusion properties on Mg(0001) surface[J]. Scientia Sinica(Technologica), 2009,39(8):1440-1447. (张健, 周惦武, 刘金水. H原子在Mg(0001)表面的吸附与扩散性能研究[J]. 中国科学: 技术科学, 2009,39(8):1440-1447.Zhang Jian, Zhou Dianwu, Liu Jinshui. Study on hydrogen atom adsorption and diffusion properties on Mg(0001) surface[J]. Scientia Sinica(Technologica), 2009, 39(8): 1440-1447. [9] Li Guangming, Gan Lihua, Chen Longwu, et al. The formation and decomposition of titanium hydride[J]. Chinese Journal of Applied Chemistry, 1998,01:77-79. (李光明, 甘礼华, 陈龙武,等. 氢化钛的制备及其分解[J]. 应用化学, 1998,01:77-79.Li Guangming, Gan Lihua, Chen Longwu, et al. The formation and decomposition of titanium hydride[J]. Chinese Journal of Applied Chemistry, 1998, 01: 77-79. [10] Wei Shengquan, Zheng Yubin, Wang Yujie, et al. First-principles calculation of geometry structures and energies of α-Ti-H system[J]. Journal of Netshape Forming Engineering, 2015(1):27-30. (魏圣泉, 郑玉斌, 王玉洁, 等. 置氢α钛几何与能量的第一性原理研究[J]. 精密成形工程, 2015(1):27-30. doi: 10.3969/j.issn.1674-6457.2015.01.005Wei Shengquan, Zheng Yubin, Wang Yujie, et al. First-principles calculation of geometry structures and energies of α-Ti-H system[J]. Journal of Netshape Forming Engineering, 2015(1): 27-30. doi: 10.3969/j.issn.1674-6457.2015.01.005 -




 下载:
下载: