Research on preparation and OER properties of vanadium doped cobalt iron layered double hydroxide
-
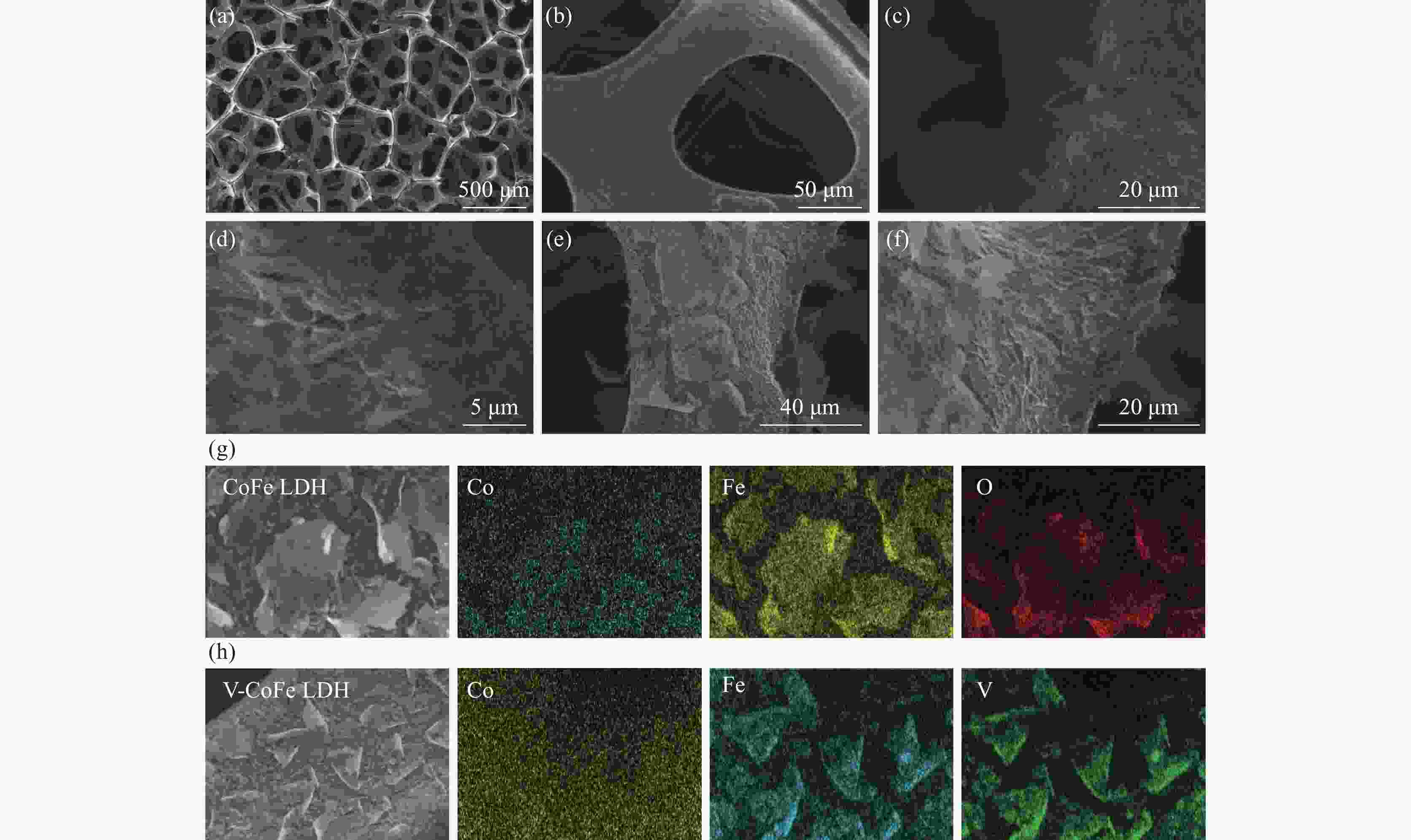
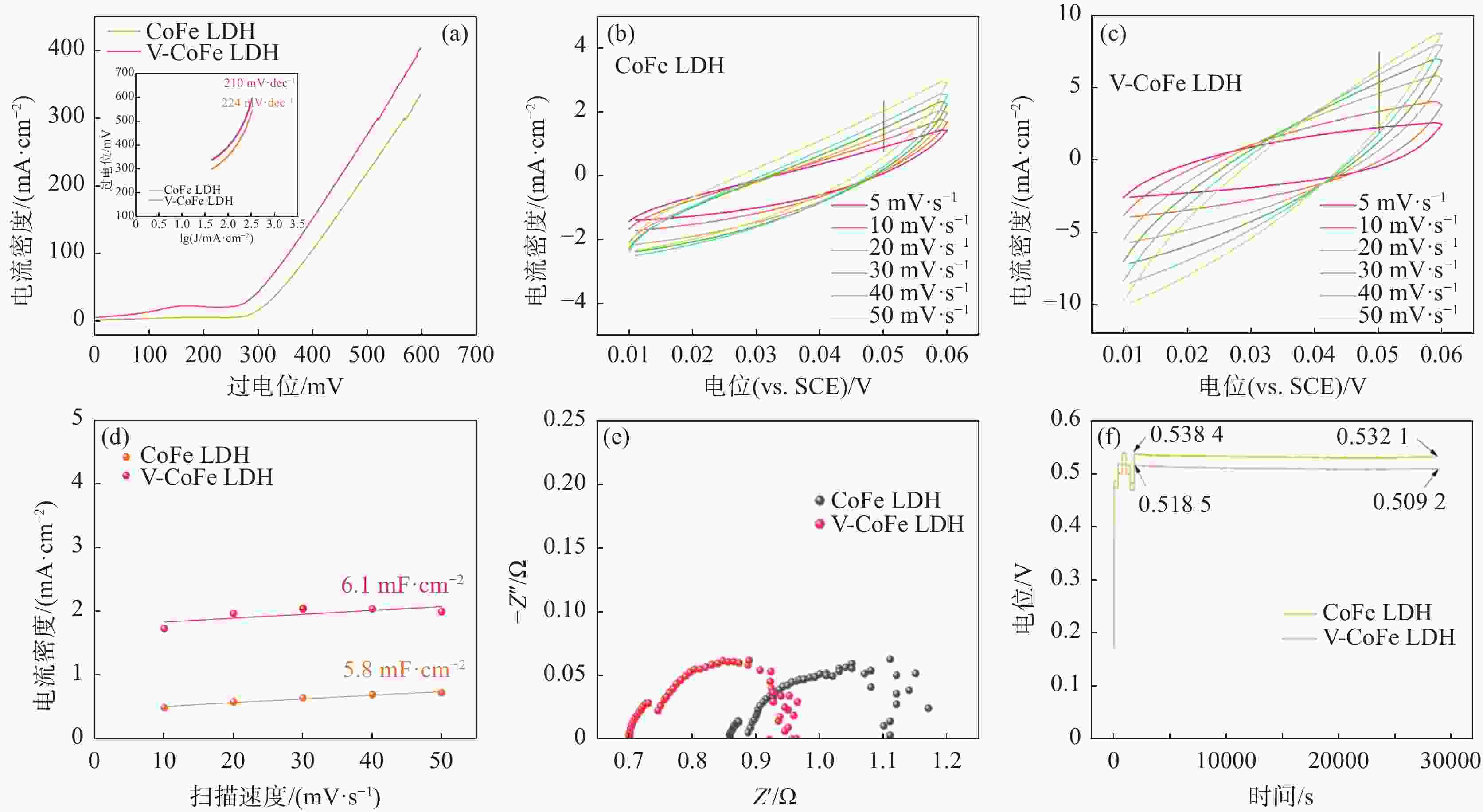
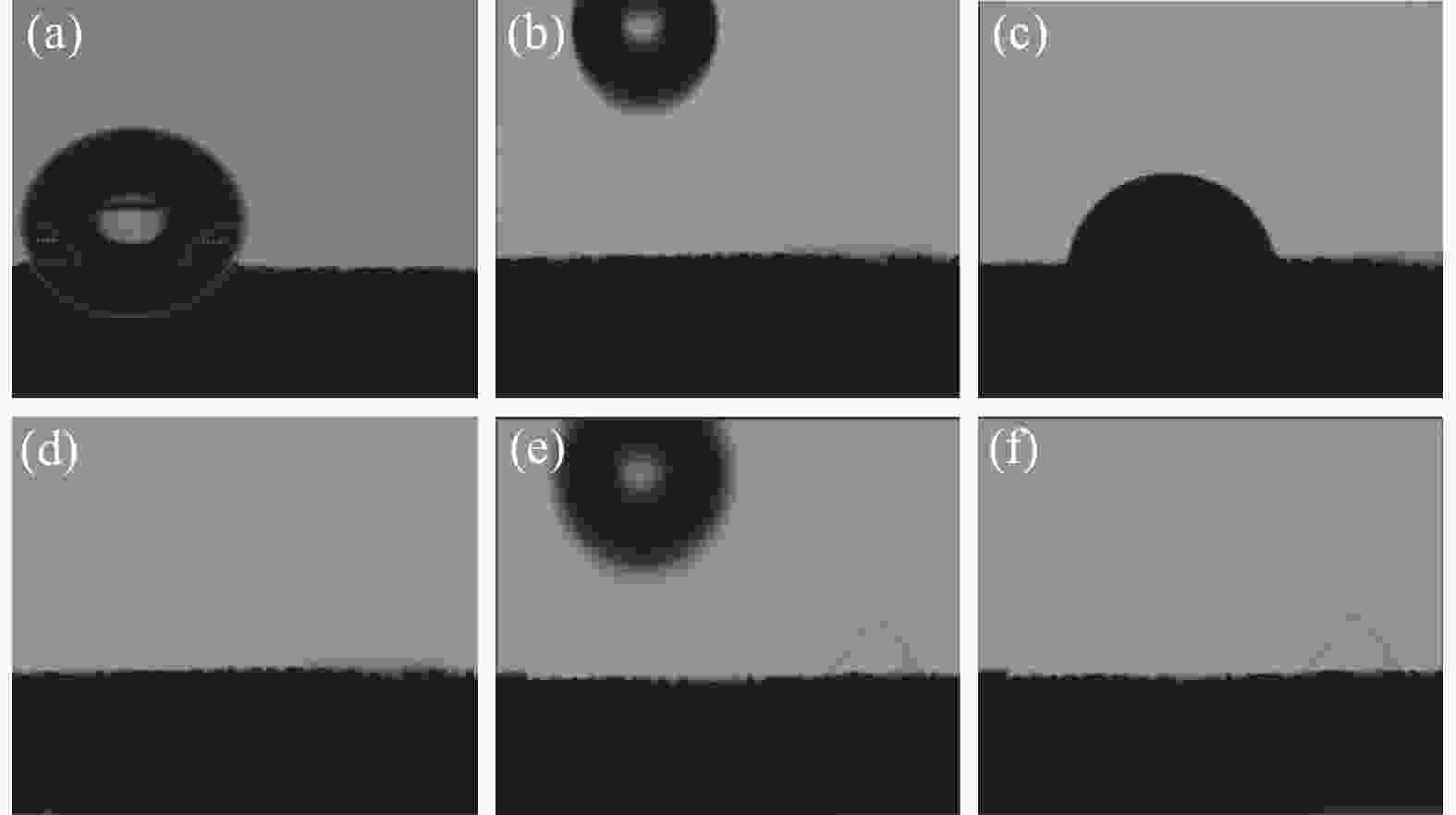
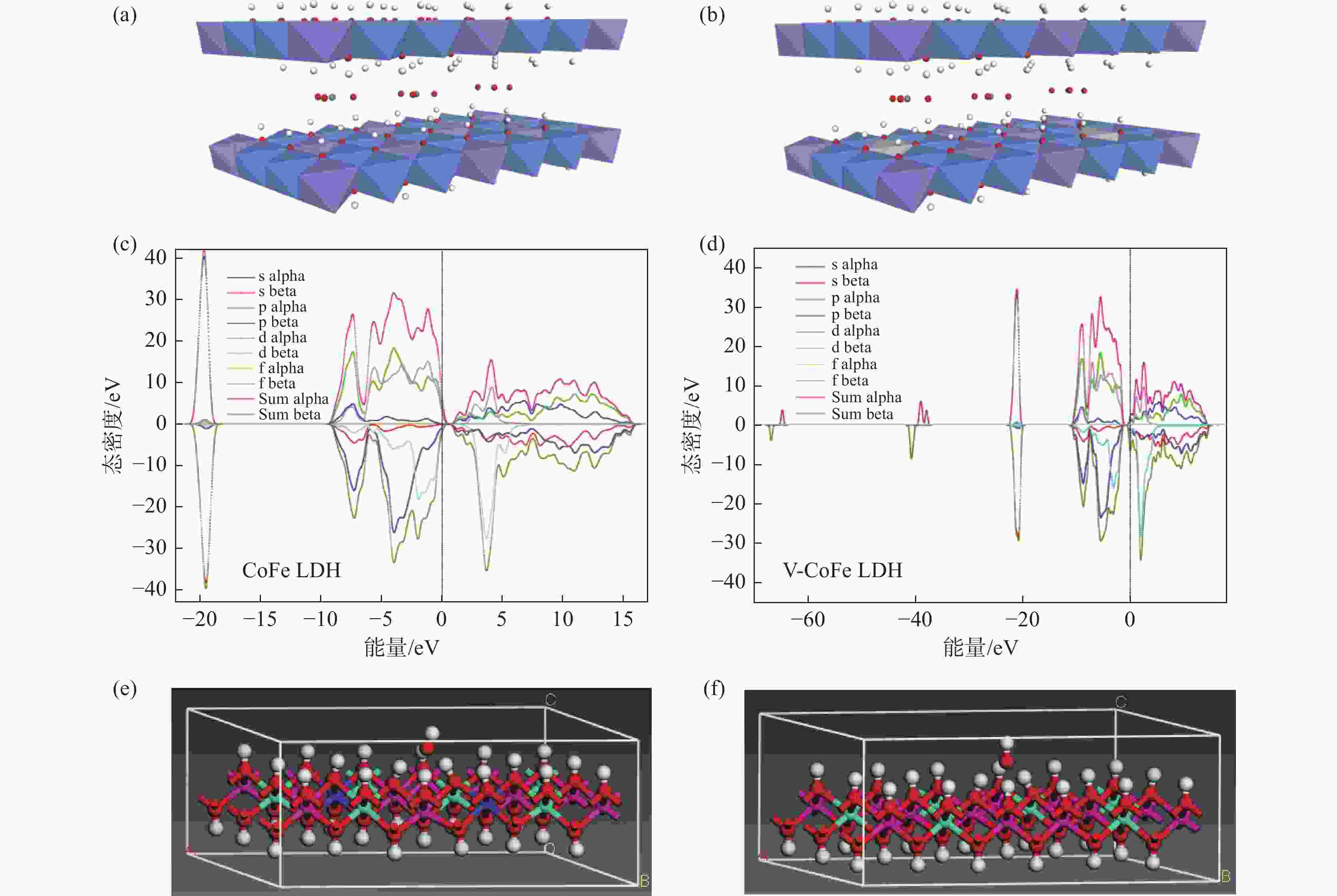
摘要: 开发环境友好且毒性相对较低的析氧反应(OER)电催化剂是目前水分解的最大困难之一。采用电沉积的方法在泡沫镍(NF)上原位生长了钴铁层状双氢氧化物(CoFe LDH)和钒掺杂的钴铁层状双氢氧化物(V-CoFe LDH)纳米片,并将其作为OER催化剂以探究其析氧性能。研究结果表明,在碱性介质中,当电流密度为100 mA·cm−2时,CoFe LDH和V-CoFe LDH的过电位分别为396 mV和356 mV,CoFe LDH和V-CoFe LDH分别具有224 mV·dec−1和210 mV·dec−1的Tafel斜率。此外,相比于CoFe LDH,V-CoFe LDH电催化剂具有大的电化学比表面积和优异的电解液润湿性。这些结果均表明V的引入有助于增强材料的OER性能。结合密度泛函理论计算和试验结果证明,V的掺杂不仅优化了材料的电子结构,增强了导电性,同样降低了吸附能,增强了催化剂与电解液的接触。Abstract: Exploiting environmentally friendly and relatively low-toxicity oxygen evolution reaction (OER) electrocatalysts is currently one of the biggest difficulties in water splitting. In this work, cobalt iron layered double hydroxide (CoFe LDH) and vanadium-doped cobalt iron layered double hydroxide (V-CoFe LDH) nanosheets are in situ grown on nickel foam (NF) by electrodeposition as an effective OER catalyst. When CoFe LDH and V-CoFe LDH samples are used as electrocatalysts, they both exhibit excellent OER performance. In an alkaline media, when the current density is 100 mA·cm−2, the overpotentials of CoFe LDH and V-CoFe LDH are small overpotentials of 396 mV and 356 mV, respectively. Tafel slopes of CoFe LDH and V-CoFe LDH are 224 mV·dec−1 and 210 mV·dec−1, respectively. The electrochemical activity specific surface area of V-CoFe LDH electrocatalysts is higher than CoFe LDH electrocatalysts. Moreover, the V-CoFe LDH electrocatalyst has better wetting properties for the electrolyte. These all indicate that the introduction of V helps to enhance the OER performance of the material. Density functional theory calculations and experimental results have shown that the doping of V not only optimizes the electronic structure of the material, enhances conductivity, but also reduces adsorption energy and enhances the contact between the catalyst and electrolyte. This work demonstrates that V-CoFe LDH is a highly promising OER electrocatalyst.
-
表 1 吸附能的DFT计算结果
Table 1. DFT calculation results of adsorption energy
eV $E_{({\mathrm{Bulk-OH}}^-)} $ $E_{({\mathrm{Bulk}})} $ $E_{{\mathrm{OH}}^-} $ $E_{{\mathrm{ads}}} $ CoFe LDH − 35140.4510 − 34685.1275 − 451.0471 − 4.2764 V-CoFe LDH − 36853.1392 − 34685.1275 − 451.0471 − 7.7834 -
[1] Wang Wei, Xu Xiaomin, Zhou Wei , et al. Recent progress in metal-organic frameworks for applications in electrocatalytic and photocatalytic water splitting[J]. Advanced Science, 2017,4(4):1600371. doi: 10.1002/advs.201600371 [2] Dincer I. Green methods for hydrogen production[J]. International Journal of Hydrogen Energy, 2012,37(2):1954-1971. doi: 10.1016/j.ijhydene.2011.03.173 [3] Lei Wanying, Zhou Tong, Pang Xin, et al. Low-dimensional MXenes as noble metal-free co-catalyst for solar-to-fuel production: Progress and prospects[J]. Journal of Materials Science & Technology, 2022,114:143-164. [4] Hu Yiming, Wang Zhaolong, Liu Wenjun, et al. A novel cobalt-iron-vanadium layered double hydroxide nanosheets arrays toward the superior water oxidation performance[J]. ACS Sustainable Chemistry & Engineering, 2019,7(19):16828-16834. [5] Reier Tobias, Mehtap Oezaslan, Peter Strasser. Electrocatalytic oxygen evolution reaction (OER) on Ru, Ir, and Pt catalysts: A comparative study of nanoparticles and bulk materials[J]. ACS Catalysis, 2012,2:1765-1772. doi: 10.1021/cs3003098 [6] Youngmin Lee, Jin Suntivich, Kevin J May, et al. Synthesis and activities of rutile IrO2 and RuO2 nanoparticles for oxygen evolution in acid and alkaline solutions[J]. Journal of Physical Chemistry Letters, 2012,3:399-404. doi: 10.1021/jz2016507 [7] Antolini Ermete. Iridium as catalyst and cocatalyst for oxygen evolution/reduction in acidic polymer electrolyte membrane electrolyzers and fuel cells[J]. ACS Catalysis, 2014,4(5):1426-1440. doi: 10.1021/cs4011875 [8] Kötz R, Lewerenz H J, Stucki S, et al. XPS studies of oxygen evolution on Ru and RuO2 anodes[J]. Journal of the Electrochemical Society, 1983,130:825-829. doi: 10.1149/1.2119829 [9] Wang Shenggao, Wang Tao, Wang Xujie, et al. Intercalation and elimination of carbonate ions of NiCo layered double hydroxide for enhanced oxygen evolution catalysis[J]. International Journal of Hydrogen Energy, 2020,23(45):12629-12640. [10] Isabela C Man, Su Haiyan, Federico Calle Vallejo, et al. Universality in oxygen evolution electrocatalysis on oxide surfaces[J]. Chem Cat Chem, 2011, 3(7): 1159-1165. [11] Long Xia, Li Jinkai, Xiao Shuang, et al. A strongly coupled graphene and FeNi double hydroxide hybrid as an excellent electrocatalyst for the oxygen evolution reaction[J]. Angewandte Chemie(International Ed), 2014, 53(29): 7584-7588. [12] Long Xia, Xiao Shuang, Wang Zilong, et al. Co intake mediated formation of ultrathin nanosheet of transition metal LDH-an advanced electrocatalysts for oxygen evolution reaction[J]. Chem Commun, 2015,1(6):1120-1123. [13] Ding Yangyang, Du Xiaoqiang, Zhang Xiaoshuang, et al. Controllable synthesis of CoFeMo layered double hydroxide nanoarrays for promoting oxygen evolution reaction[J]. Dalton Transactions, 2020,49:15417-15424. doi: 10.1039/D0DT03182H [14] Gong Ming, Li Yanguang, Wang Hailiang, et al. An advanced NieFe layered double hydroxide electrocatalyst for water oxidation[J]. Journal of the American Chemical Society, 2013,135(23):8452-8455. doi: 10.1021/ja4027715 [15] Yu Xiaowen, Zhang Miao, Yuan Wenjing, et al. High-performance three-dimensional Ni-Fe layered double hydroxide/graphene electrode for water oxidation[J]. Journal of Materials Chemistry A, 2015,3(13):6921-6928. doi: 10.1039/C5TA01034A [16] Li Kaiyue, Guo Dong, Kang Jianyu, et al. Hierarchical hollow spheres assembled with ultrathin CoMn double hydroxide nanosheets as trifunctional electrocatalyst for overall water splitting and Zn air battery[J]. ACS Sustainable Chemistry & Engineering, 2018,6(11):14641-14651. [17] Yang Yang, Dang Lianna, Shearer Melinda J, et al. Highly active trimetallic NiFeCr layered double hydroxide electrocatalysts for oxygen evolution reaction[J]. Advanced Energy Materials, 2018,8(15):1703189. doi: 10.1002/aenm.201703189 [18] Feng Yihan, Li Zichuang, Li Shanlin, et al. One stone two birds: Vanadium doping as dual roles in self-reduced Pt clus-ters and accelerated water splitting[J]. Journal of Energy Chemistry, 2022,66:493-501. doi: 10.1016/j.jechem.2021.08.061 [19] Wang Shenggao, Wang Tao, Wang Xujie, et al. Intercalation and elimination of carbonate ions of NiCo layered double hydroxide for enhanced oxygen evolution catalysis[J]. International Journal of Hydrogen Energy, 2020,45(23):12629-12640. doi: 10.1016/j.ijhydene.2020.02.212 [20] Wang Bo, Gareth R Williams, Chang Zheng, et al. Hierarchical NiAl layered double hydroxide/multiwalled carbon nanotube/nickel foam electrodes with excellent pseudocapacitive properties[J]. ACS Applied Materials & Interfaces, 2014,6:16304-16311. [21] Wang Yuhang, Chen Long, Yu Xiaomin, et al. Superb alkaline hydrogen evolution and simultaneous electricity generation by Pt-decorated Ni3N nanosheets[J]. Advanced Energy Materials, 2016,7:1601390. [22] Tian Yang, Bi Yongming, Qin Bangchang, et al. Density functional theory investigation of oxygen evolution reaction on the NiFe-LDHs (100) surface[J]. Joural of Advances in Physical Chemistry, 2017,6(2):75-83. (田阳, 毕永民, 秦邦昌, 等. NiFe-LDHs催化氧气析出反应的密度泛函理论研究[J]. 物理化学进展, 2017,6(2):75-83. doi: 10.12677/JAPC.2017.62010Tian Yang, Bi Yongming, Qin Bangchang, et al. Density functional theory investigation of oxygen evolution reaction on the NiFe-LDHs (100) surface[J]. Joural of Advances in Physical Chemistry, 2017, 6(2): 75-83. doi: 10.12677/JAPC.2017.62010 -




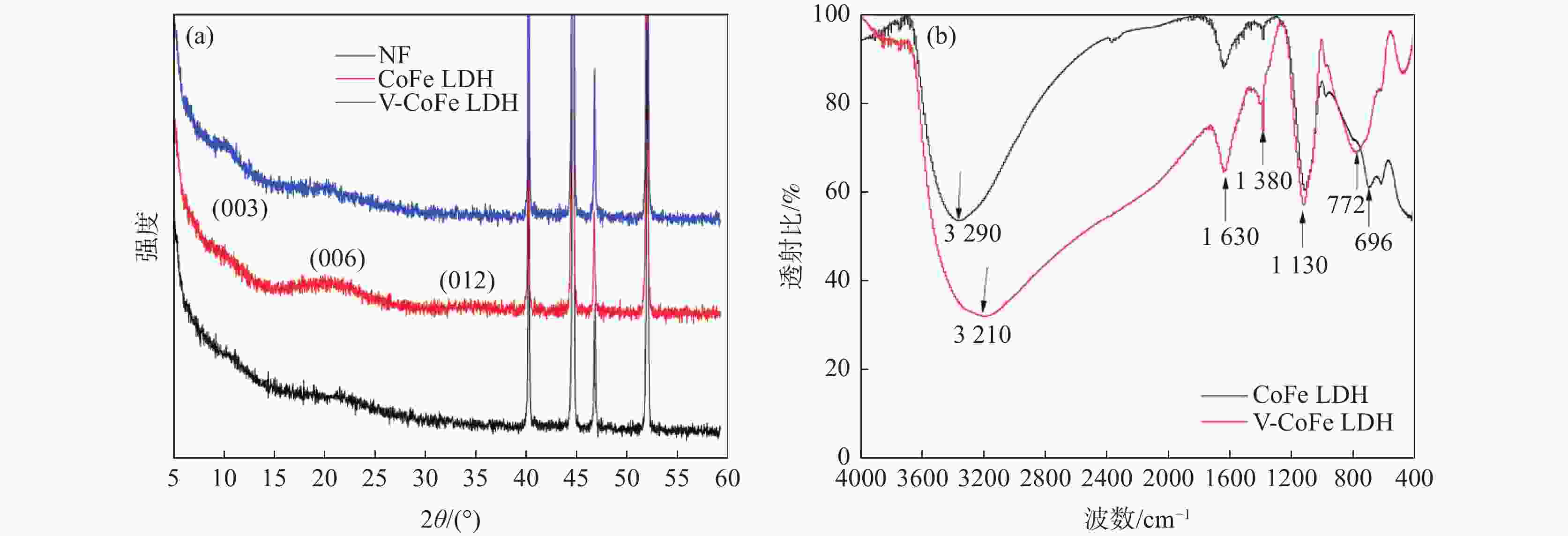
 下载:
下载: