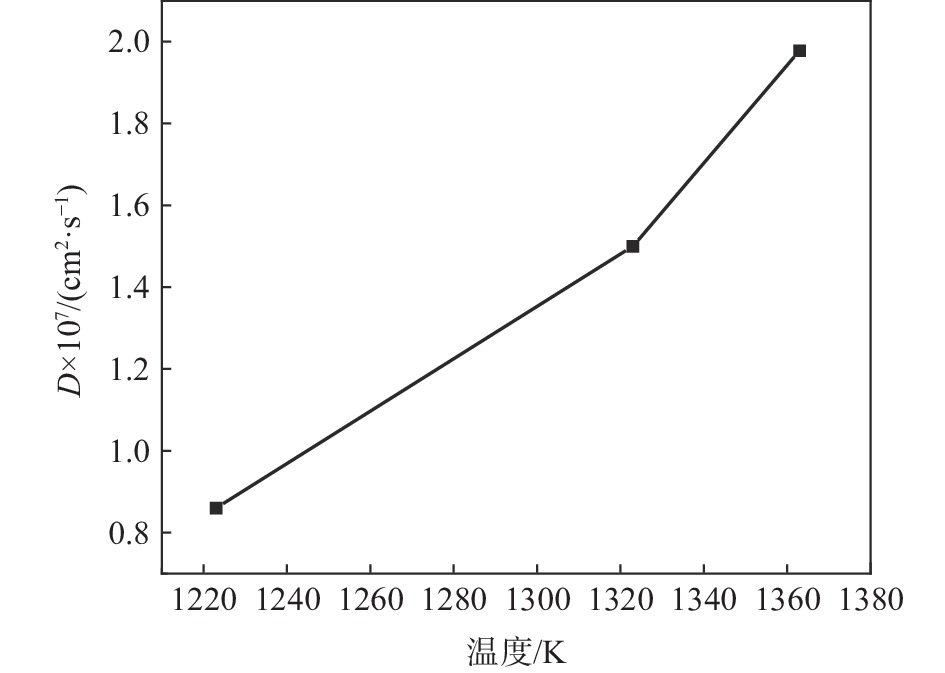
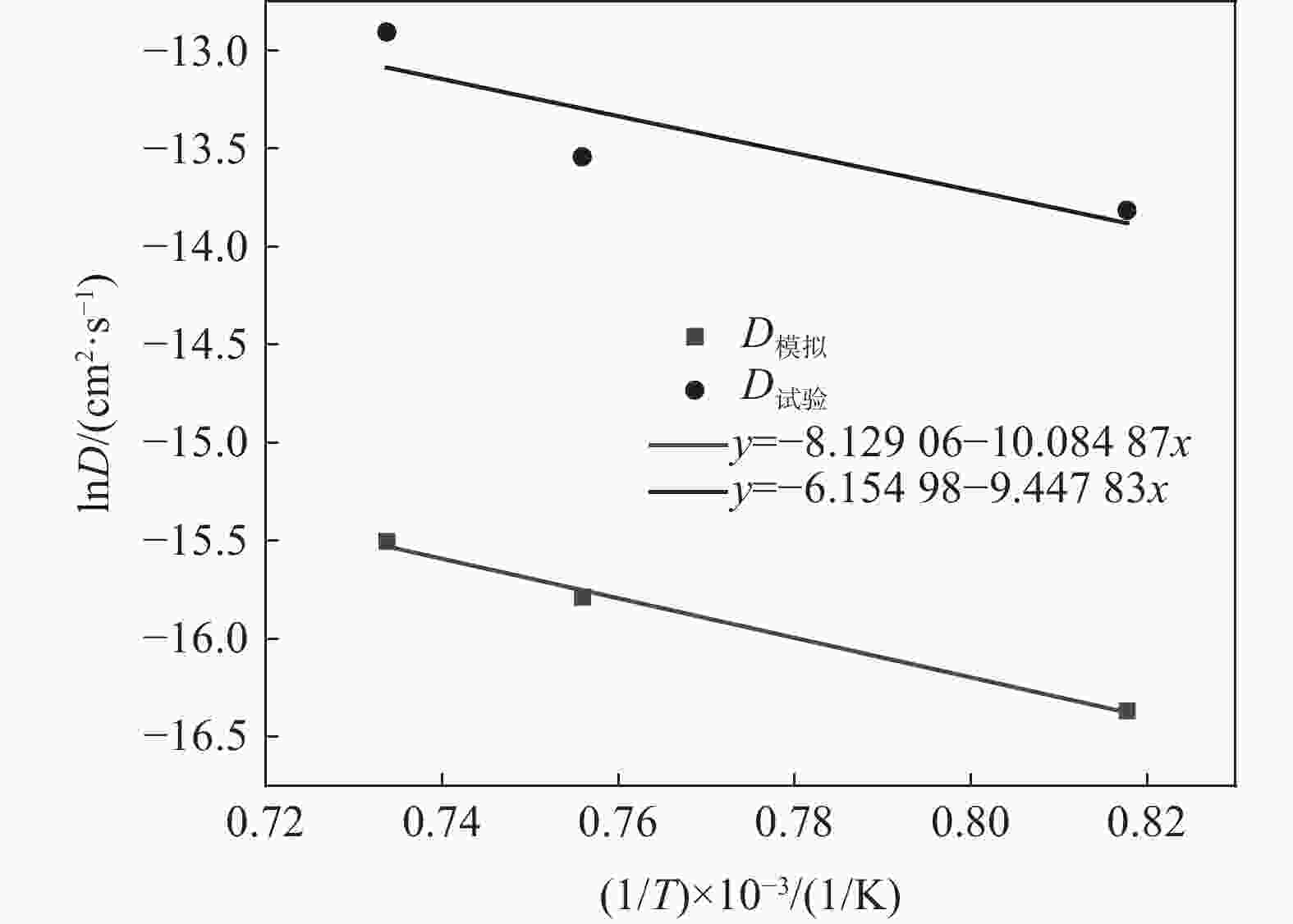
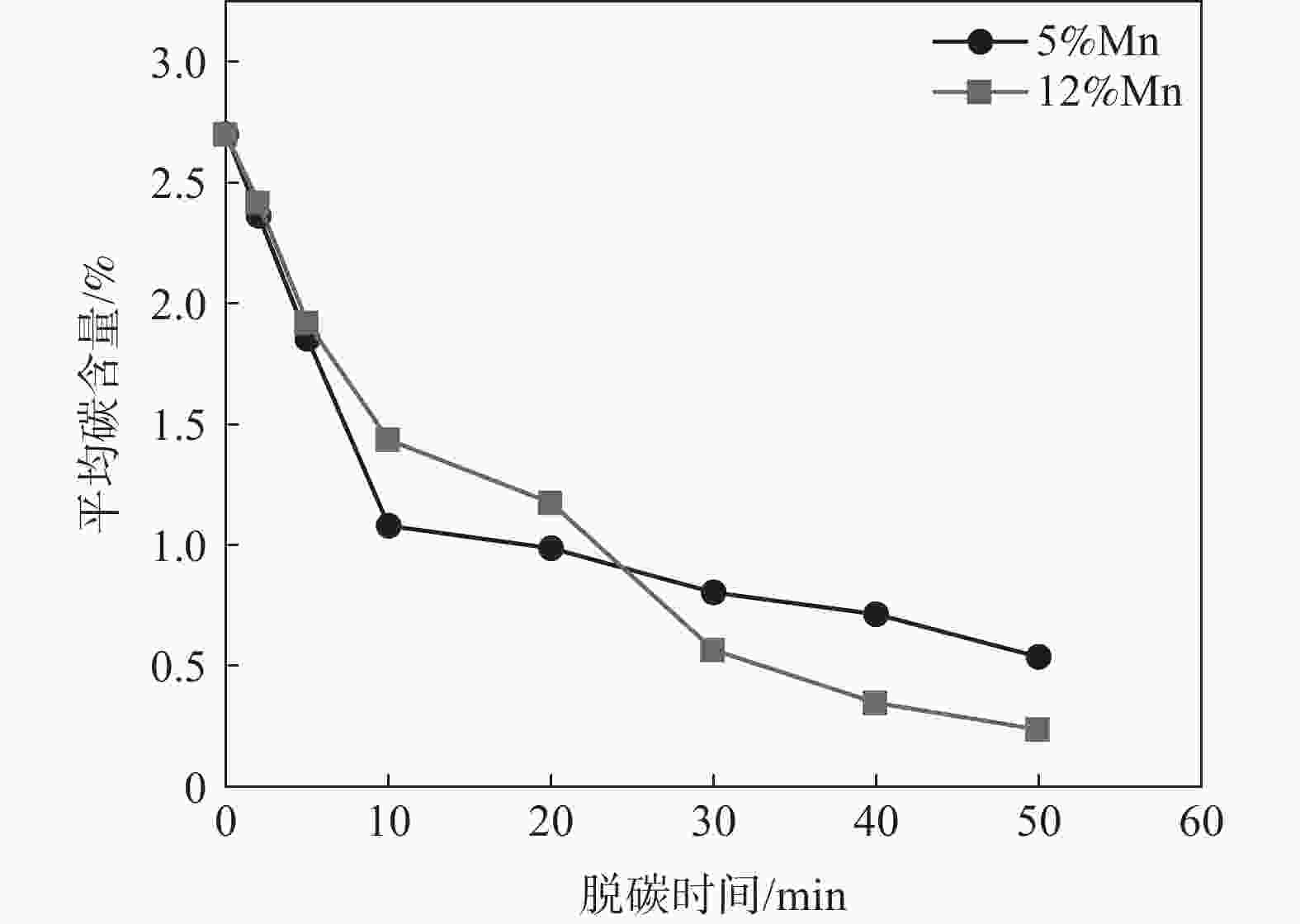
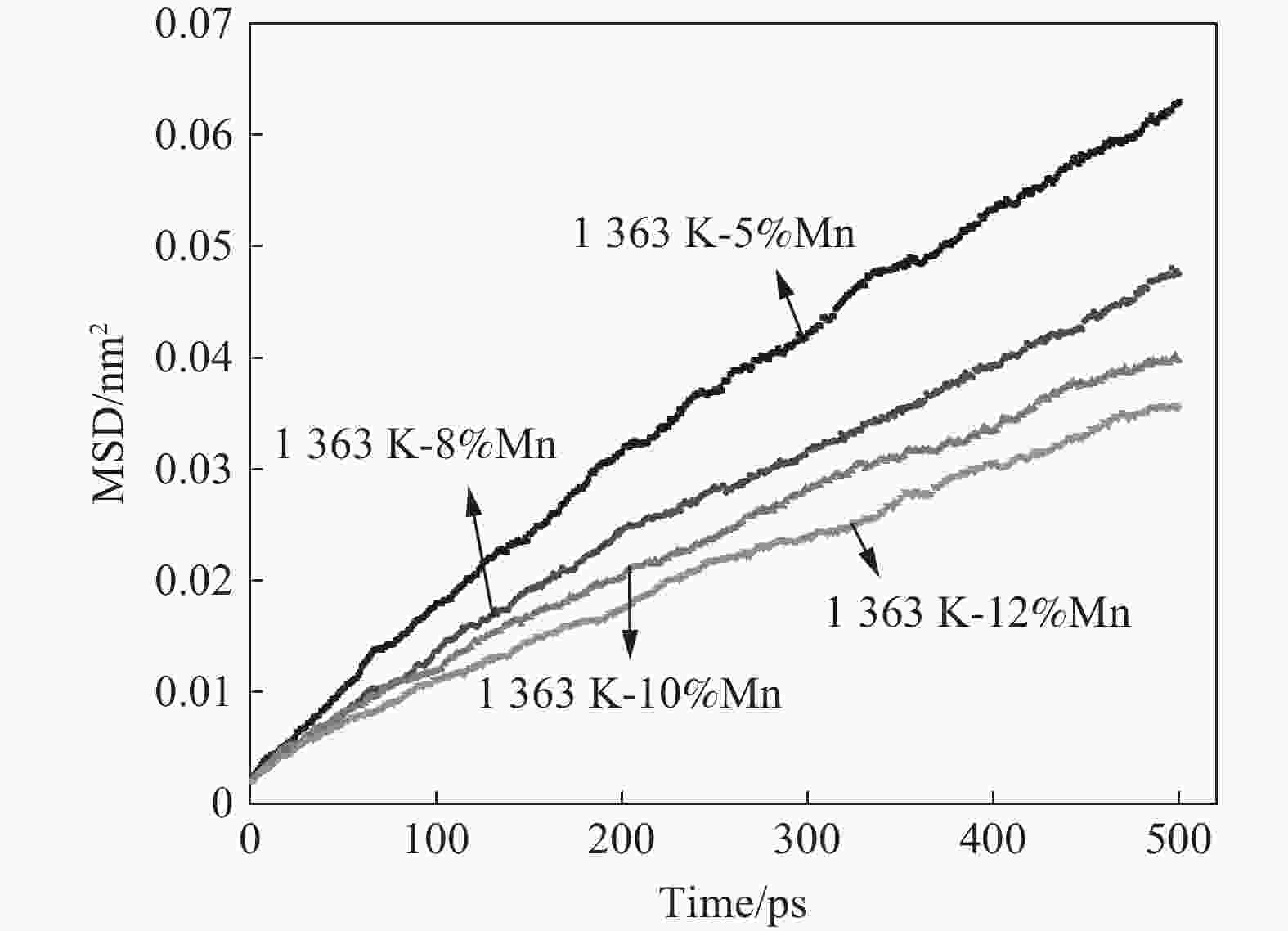
| [1] |
Li Yaqiang, Ai Liqun. The proposal and progress of new process research on solid-state steelmaking[J]. Foundry Technology, 2017,38(1):8-11. (李亚强, 艾利群. 固态炼钢新工艺研究的提出与进展[J]. 铸造技术, 2017,38(1):8-11.Li Yaqiang, Ai Liqun. The proposal and progress of new process research on solid-state steelmaking[J]. Foundry Technology, 2017, 38(1): 8-11.
|
| [2] |
Hong Lukuo, Ai Liqun. New process of solid steelmaking[J]. Industrial Metrology, 2015,25(S1):161-163. (洪陆阔, 艾立群. 浅谈固态炼钢新工艺[J]. 工业计量, 2015,25(S1):161-163.Hong Lukuo, Ai Liqun. New process of solid steelmaking[J]. Industrial Metrology, 2015, 25(S1): 161-163.
|
| [3] |
Park J O, Long T V, Sasaki Y. Feasibility of solid-state steelmaking from cast iron decarburization of rapidly solidified cast iron[J]. ISIJ International, 2012,98(5):151-160.
|
| [4] |
Lee W H, Park J O, Lee J S, et al. Solid state steelmaking by decarburisation of rapidly solidified high carbon iron sheet[J]. Ironmaking & Steelmaking, 2012,39(7):530-534.
|
| [5] |
Sharif S E, Mirjalili M, Khaki J V. A new approach in solid state steelmaking from thin cast iron sheets through decarburization in CaCO3 pack[J]. ISIJ International, 2018,58(10):1791-1800. doi: 10.2355/isijinternational.ISIJINT-2018-250
|
| [6] |
Sun Caijiao, Ai Liqun, Hong Lukuo, et al. Decarburization kinetics of iron-carbon alloy ribbons by gas-solid reaction in Ar-CO-CO2 atmosphere[J]. Materials Reports, 2021,35(24):24142-24146. (孙彩娇, 艾立群, 洪陆阔, 等. Ar-CO-CO2气氛下铁碳合金薄带气固反应脱碳动力学研究[J]. 材料导报, 2021,35(24):24142-24146.Sun Caijiao, Ai Liqun, Hong Lukuo, et al. Decarburization kinetics of iron-carbon alloy ribbons by gas-solid reaction in Ar-CO-CO2 atmosphere[J]. Materials Reports, 2021, 35(24): 24142-24146.
|
| [7] |
Sun Caijiao, Ai Liqun, Hong Lukuo, et al. Decarburization mechanism of Fe-C alloy in H2 / H2O atmosphere by gas-solid reaction[J]. Materials Reports, 2020,34(20):20112-20117. (孙彩娇, 艾立群, 洪陆阔, 等. H2/H2O气氛下Fe-C合金气固反应脱碳机理[J]. 材料导报, 2020,34(20):20112-20117.Sun Caijiao, Ai Liqun, Hong Lukuo, et al. Decarburization mechanism of Fe-C alloy in H2 / H2O atmosphere by gas-solid reaction[J]. Materials Reports, 2020, 34(20): 20112-20117.
|
| [8] |
Chen Pengfei, Ai Liqun. Study on gas-solid reaction decarburization of medium carbon domain iron-carbon alloy ribbons[J]. Iron Steel Vanadium Titanium, 2020,41(3):105-109. (陈鹏飞, 艾立群. 中碳域铁碳合金薄带气—固反应脱碳研究[J]. 钢铁钒钛, 2020,41(3):105-109.Chen Pengfei, Ai Liqun. Study on gas-solid reaction decarburization of medium carbon domain iron-carbon alloy ribbons[J]. Iron Steel Vanadium Titanium, 2020, 41(3): 105-109.
|
| [9] |
Hong Lukuo. Temperature and atmosphere control of gas-solid reaction decarburization of iron-carbon alloy ribbons[D]. Tangshan: North China University of Science and Technology, 2015. (洪陆阔. 铁碳合金薄带气—固反应脱碳温度与气氛控制[D]. 唐山:华北理工大学2015.Hong Lukuo. Temperature and atmosphere control of gas-solid reaction decarburization of iron-carbon alloy ribbons[D]. Tangshan: North China University of Science and Technology, 2015.
|
| [10] |
Cheng Rong. Kinetic analysis of gas-solid reaction decarburization of iron-carbon alloy ribbons[D]. Tangshan: North China University of Science and Technology, 2016. (程荣. 铁碳合金薄带气—固反应脱碳动力学分析[D]. 唐山:华北理工大学, 2016.Cheng Rong. Kinetic analysis of gas-solid reaction decarburization of iron-carbon alloy ribbons[D]. Tangshan: North China University of Science and Technology, 2016.
|
| [11] |
Hou Yaobin. Decarburization of Fe-C alloy by step heating in CO-CO2 atmosphere[D]. Tangshan: North China University of Science and Technology, 2021. (侯耀斌. CO-CO2气氛下分段加热对Fe-C合金脱碳研究[D]. 唐山:华北理工大学, 2021.Hou Yaobin. Decarburization of Fe-C alloy by step heating in CO-CO2 atmosphere[D]. Tangshan: North China University of Science and Technology, 2021.
|
| [12] |
Lee B J. A modified embedded-atom method interatomic potential for the Fe–C system[J]. Acta Materialia, 2006,54(3):701-711. doi: 10.1016/j.actamat.2005.09.034
|
| [13] |
Kim Y M, Shin Y H, Lee B J. Modified embedded-atom method interatomic potentials for pure Mn and the Fe–Mn system[J]. Acta Materialia, 2009,57(2):474-482. doi: 10.1016/j.actamat.2008.09.031
|
| [14] |
Rappé A K, Casewit C J, Colwell K S, et al. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations[J]. Journal of the American Chemical Society, 1992,114(25):10024-10035. doi: 10.1021/ja00051a040
|
| [15] |
Wang Shaogang, Liu Cuixia, Jian Zengyun. Molecular dynamics simulation of diffusion coefficient of Al-Cu alloy[J]. Journal of Xi'an Technological University, 2018,38(6):559-564. (王少刚, 刘翠霞, 坚增运. Al-Cu合金扩散系数的分子动力学模拟研究[J]. 西安工业大学学报, 2018,38(6):559-564.Wang Shaogang, Liu Cuixia, Jian Zengyun. Molecular dynamics simulation of diffusion coefficient of Al-Cu alloy[J]. Journal of Xi'an Technological University, 2018, 38(6): 559-564.
|
| [16] |
Li Yaqiang, Ai Liqun, Li Qiang, et al. 1 mm iron-carbon alloy strip gas-solid reaction decarburization test[J]. Iron & Steel, 2017,52(5):19-23, 35. (李亚强, 艾立群, 李强, 等. 1 mm铁碳合金薄带气—固反应脱碳试验[J]. 钢铁, 2017,52(5):19-23, 35.Li Yaqiang, Ai Liqun, Li Qiang, et al. 1 mm iron-carbon alloy strip gas-solid reaction decarburization test[J]. Iron & Steel, 2017, 52(5): 19-23, 35.
|
| [17] |
Li Yaqiang. Effect of atmosphere conditions on gas-solid reaction decarburization of iron-carbon alloy ribbons[D]. Tangshan: North China University of Science and Technology, 2017. (李亚强. 气氛条件对铁碳合金薄带气—固反应脱碳的影响[D]. 唐山:华北理工大学, 2017.Li Yaqiang. Effect of atmosphere conditions on gas-solid reaction decarburization of iron-carbon alloy ribbons[D]. Tangshan: North China University of Science and Technology, 2017.
|
| [18] |
Meng Fanjun, Ai Liqun, Hong Lukuo, et al. Experimental study on decarburization of Fe-C-Mn ribbons in Ar-H2O-H2 atmosphere[J]. Iron Steel Vanadium Titanium, 2021,42(5):132-137. (孟凡峻, 艾立群, 洪陆阔, 等. Ar-H2O-H2气氛下Fe-C-Mn薄带脱碳试验研究[J]. 钢铁钒钛, 2021,42(5):132-137.Meng Fanjun, Ai Liqun, Hong Lukuo, et al. Experimental study on decarburization of Fe-C-Mn ribbons in Ar-H2O-H2 atmosphere[J]. Iron Steel Vanadium Titanium, 2021, 42(5): 132-137.
|
| [19] |
Sun Caijiao, Ai Liqun, Hong Lukuo, et al. Study on solid state steelmaking from thin cast iron sheets through decarburization in H2O-H2[J]. Ironmaking & Steelmaking, 2020,47(9):1015-1021.
|
| [20] |
Král L, Million B, Čermák J. Diffusion of carbon and manganese in Fe-C-Mn[C]//Defect and Diffusion Forum. Trans Tech Publications Ltd, 2007, 263: 153-158.
|




 下载:
下载: